Question #450ff
1 Answer
Giant Tetrahedral structure and covalent bonding.
Explanation:
Most materials in the world have molecules that are all attached to each other in small clusters.
And due to intermolecular forces
(if you don't know about it, it's a subject of its own, so here is a video explaining it) the molecules attach to each other pretty weakly. Some heat or contact can break these bonds, aka the structure of the materials.
Their structures are therefore usually more random and easy to mess with. As an analogy, it's like building a house out of sand or mud.
 http://www.keywordsuggests.com
http://www.keywordsuggests.com
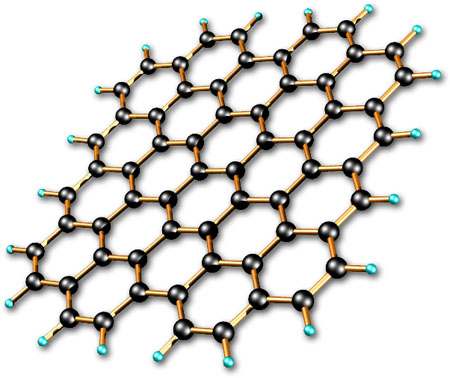
But diamonds, graphene, and carbon nanotubes all use this structure where the carbon atoms are all directly linked to each other aka network covalent, (the strongest type of bond) which gives them a really strong structure. So if you think about it, a diamond is just one big molecule.
This is like building a house out of bricks, which is clearly much stronger than sand.
 Pinterest
Pinterest
The way graphene carbon atoms are attached to each other are very much like diamond then, just as a sheet instead of a crystal.


