What family has the lowest ionization energy?
1 Answer
May 10, 2017
Explanation:
Group 1 of the Periodic Table features metals whose valence electron SINGLY occupies the outermost shell, the valence shell of the given atoms. Since the nuclear charge is necessarily diminished with respect to the valence shell, the alkali metals display the lowest ionization energies, and these energies (reasonably) decrease down the Group.
But a chemist, a physical scientist should examine the data.
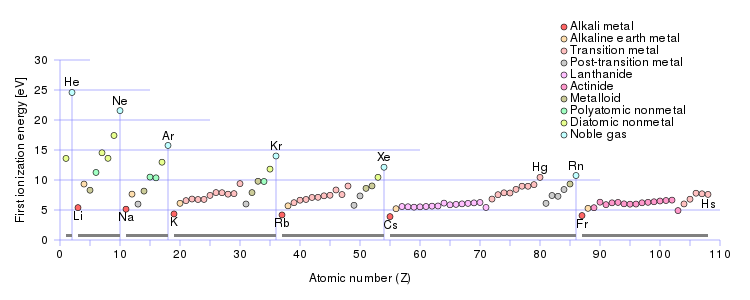
And here the first ionization energy is plotted against the atomic number, and the respective ionization energies of the alkali metal series are clearly represented. Do these data support what I have said? Why or why not.

