Question #b9f95
1 Answer
b) There can be isomeric pairs in (i) alcohols and ethers and in (ii) aldehydes and ketones.
Explanation:
a) Alkanes and cycloalkanes
These are not isomeric with each other.
Compare propane (
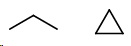
They cannot be isomers because they have different molecular formulas.
The general formula for alkanes is
b) Oxygen-containing compounds
The classes are not isomeric with each other.
However, members from each class with the same number of carbon atoms can be isomers, depending on the position of the oxygen atom.
(i) Alcohols and ethers
Compare ethanol

They are isomers because they have the same chemical formula but different arrangements of their atoms.
The general formula of alcohols and ethers is
(ii) Aldehydes and Ketones
Compare propanal and
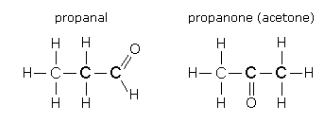
They are isomers because they have the same chemical formula but different arrangements of their atoms.
The general formula of alcohols and ethers is

