Why do ideal solutions behave differently from non-ideal solutions?
1 Answer
You are asking why black bears are not brown bears,,,,,,
Explanation:
Ideal solutions obey Raoult's Law:
Azeotropes are constant boiling mixtures that distil without change in composition. Azeotropes can show positive variation from
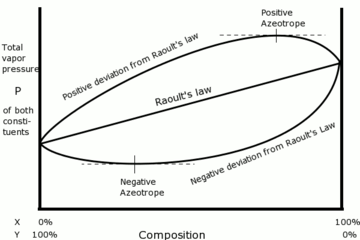
And negative variation shows greater (attractive) interaction between different solvent molecules, and positive deviation shows greater repulsive interaction. Non-ideality is indicated when the solution vapour pressure departs from linearity as shown on the graph.
So the answer to why black bears are not brown bears is that they are different categories.

