The order of the elements in the modern periodic table is based on what aspect of the element?
2 Answers
Explanation:
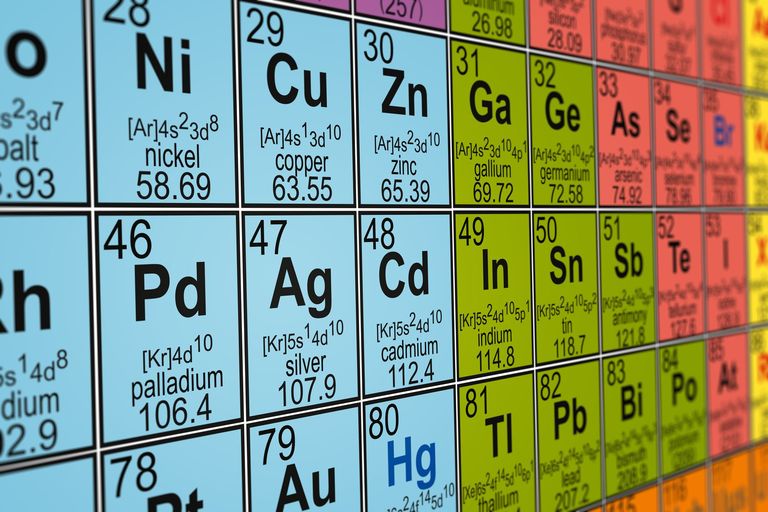
By "order of the elements", I assume that you are talking about the left-to-right rows, or periods, of the periodic table. In that case, the atomic mass determines this order. For instance, Hydrogen's atomic mass is 1.008, Helium's is 4.003, Lithium's is 6.941, and so on.
The elements in the modern periodic table are arranged in order of their atomic numbers, which is the number of protons in the nuclei of the atoms of an element.
Explanation:
The elements in the modern periodic table are arranged in order of their atomic numbers, which is the number of protons in the nuclei of the atoms of an element. Each element has a unique atomic number. The atomic numbers are also whole numbers.
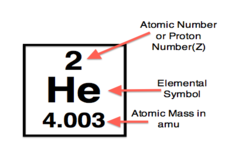
Notice that the elements are arranged in order of their atomic numbers, which are whole numbers and in sequence.