Question #4bc39
1 Answer
The molecule you are looking for is probably benzene,
Explanation:
First, we use the given percentages to estimate how many carbon and hydrogen atoms we have in our molecule.
Since the mass of one carbon atom is 12.01 u, and the mass of one hydrogen atom is 1.008 u, we can write:
We fill in the percentages for the two atoms and take 80 for the molecular mass at first. We obtain:
Now we have a starting point to look for our molecule. We now know that we probably have the same amount of hydrogen atoms and carbon atoms.
We first try to justify a molecular formula for this composition. This just means that we don't exceed our limit of the molecular weight
We try
Therefore we will make structures with molecular formula
The simplest example that I can think of is benzene, shown below.
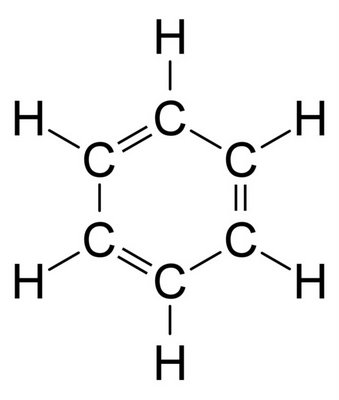
It is always good to check your answer if you have the time, you could, for example, calculate the percentages from 78 again, which in this case will give you the exact percentages back from the question.
