What is the nuclide symbol and nuclide name described by the following particle: 92 protons and 146 neutrons?
1 Answer
Uranium-238.
Explanation:
In order to find the identity of the nuclide, you need to know two things
- the atomic number of the atom
- the mass number of the atom
The atomic number,
The mass number,
#color(blue)(ul(color(black)(A = Z + "no. of neutrons")))#
In your case, the mass number of the nuclide is equal to
#A = 92 + 146 = 238#
Now, isotope notation makes use of the atomic number and the mass number of the nuclide.
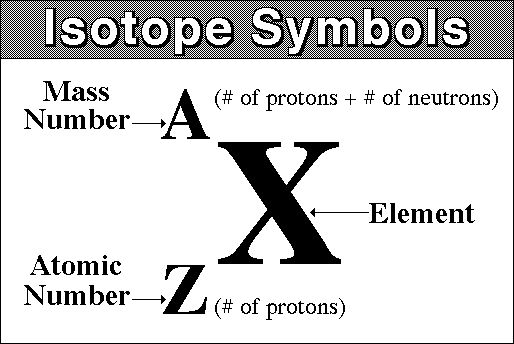
The identity of the nuclide is given by the atomic number, so grab a periodic table and look for the element that has
This element is uranium,
uranium-238
Consequently, you can say that the nuclide symbol will be
#""_(color(white)(1)92)^238"U" -># uranium-238

