During a phase change, what happens to the temperature of a substance?
1 Answer
Jun 7, 2017
We don't really know, because there are two common types of phase changes...
Consider the phase diagram for
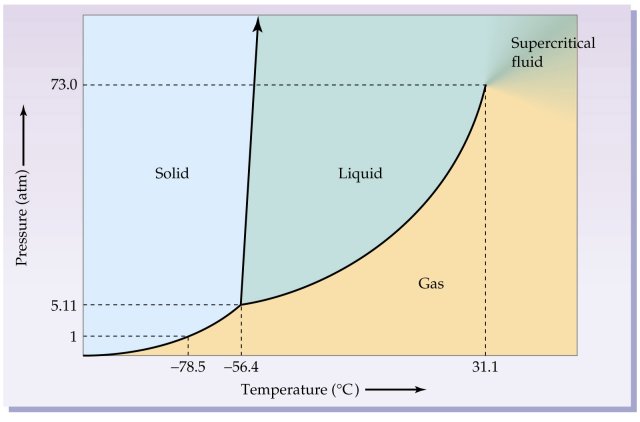
This says that a sublimation phase change could happen, for example, at a constant
That is a horizontal phase transition, with a change in temperature at constant pressure.
But we could also keep the temperature constant at
That is a vertical phase transition, with a change in pressure at constant temperature.

