For which phase is entropy lowest? Highest?
1 Answer
Jun 19, 2017
Entropy describes the amount of energy dispersal in a system. In other words, it is proportional to the capacity the system has to spread energy throughout it.
Basically, it increases with the amount of motion in the system. The more motion there is, the more energy is in the system in the first place, and thus the more energy that can be dispersed or spread throughout the system. This would indicate a higher entropy.
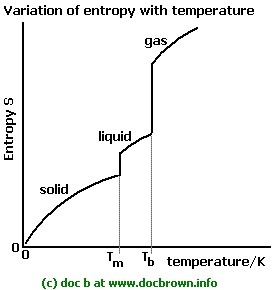
The system entropy is therefore in general greatest for gases and lowest for solids.
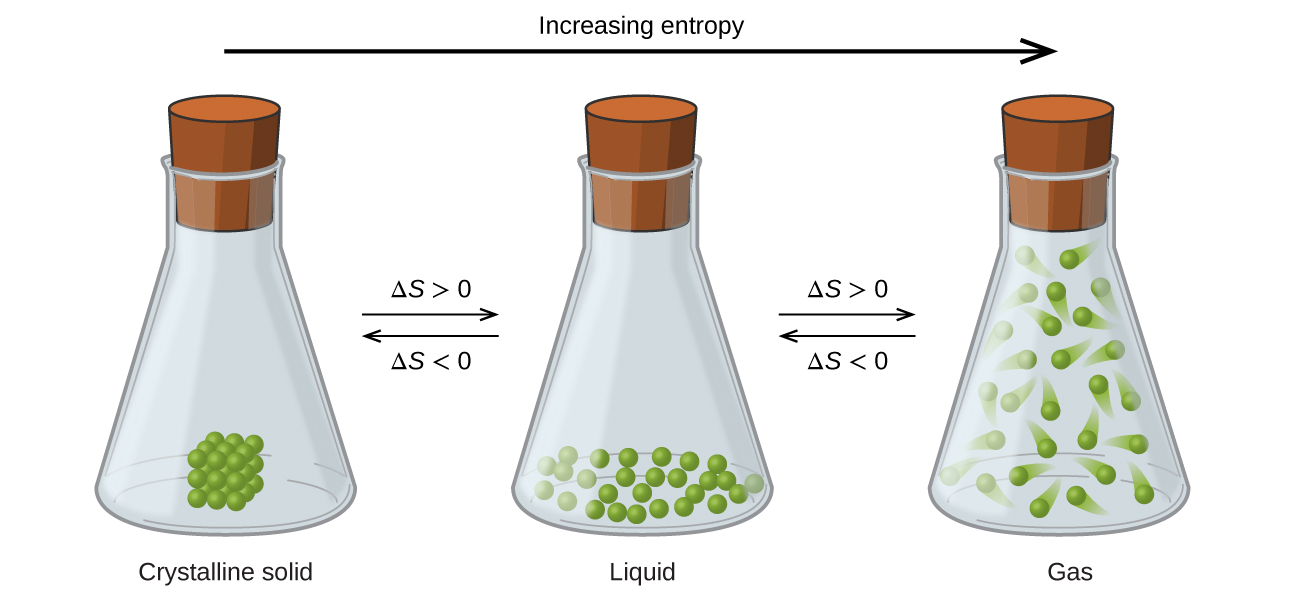
And as a result of the above trend, we can also say that the change in entropy for melting and vaporization are positive, and the change in entropy for freezing and condensation are negative.

