Why do transition elements exhibit such a variety of coloured compounds, complexes and different oxidation states?
1 Answer
Jun 21, 2017
It is mainly due to the closeness of the energy levels of the
- The variety of colored complexes is due to the various oxidation states possible; a different oxidation state on the central metal atom alters the metal-ligand
#d# -orbital splitting energy, which gives rise to varied emission wavelengths. - Oxidation states vary due to the general ease of incorporating the
#bb((n-1)d)# electrons into bonding.
And the general ease of incorporating the
(You can see it's all connected.)
To illustrate this, I took orbital potential energy data from here (Appendix B.9) to graph the orbital potential energies for the
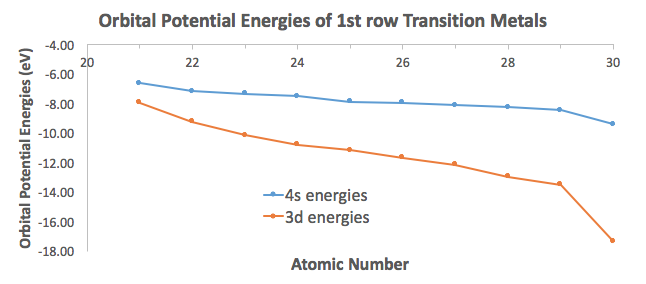
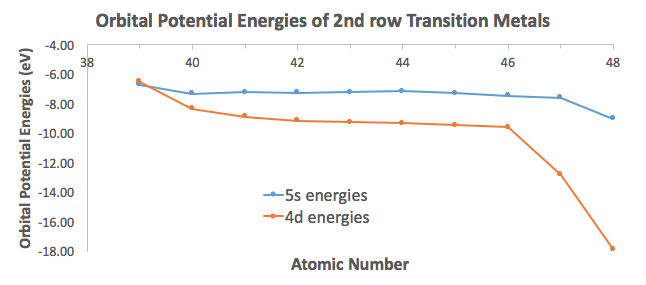
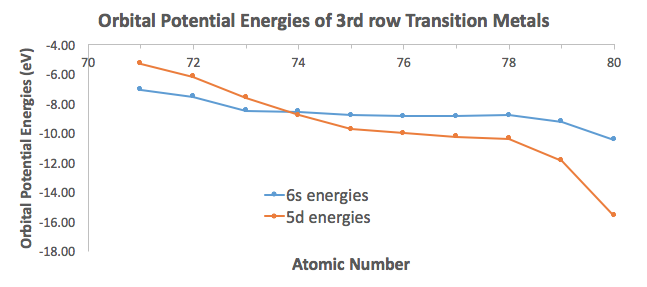
For the most part, the difference in energies are within

