See this old answer. Now isomers are materials of the #"SAME chemical formula"#, but................
#"DIFFERENT CONNECTIVITY OR GEOMETRY."#
#"Structural isomers"# have different connectivity, and structural isomerism is exemplified by a wealth of carbon chemistry. Consider #"butane"#, #C_4H_10#; this formula can support two STRUCTURAL isomers, #H_3C-CH_2CH_2CH_3#, #"n-butane"#, or #H_3C-CH(CH_3)CH_3#, #"isobutane"#. And as the formula grows larger, i.e. #"pentane"#, #"hexane"#, the number of possible structural isomers substantially grows.
On the other hand, #"geometric isomers"# are isomers of the same connectivity, but #"DIFFERENT GEOMETRY"#. And possibly the best exemplar is #"cis"# versus #"trans-butylene"#.
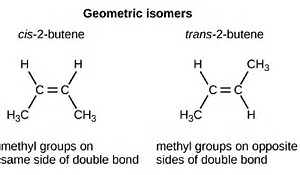
Now note that for either isomer, the carbon-carbon chain is IDENTICAL with respect to connectivity; are you clear on this? #C_1# connects to #C_2# connects to #C_3# connects to #C_4#. Nevertheless, because the geometry of the chain is different, these isomers have different physical and chemical properties. The normal boiling points of the #"cis"# and #"trans"# isomers are #3.7# #""^@C#, and #0.9# #""^@C# respectively.


