What is an explanation as to why the ionization energy changes as it does for other periods and groups?
1 Answer
The ionization energy reflects two competing properties...........
Explanation:
So what you gots to remember. Ionization energy INCREASES across a Period, from left to right as we face the Table. Ionization energy DECREASES down a Group, a column of the Periodic Table.
Remember we interrogate the reaction.......
And as chemists, as physical scientists, we should consider actual data......
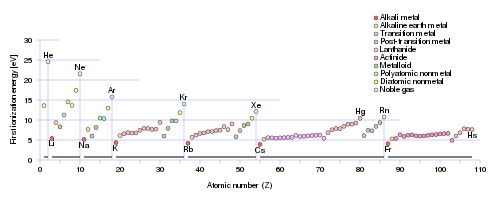
Does this graph support what I have argued? Why or why not? Why should the Noble Gases have the largest ionization energy of their Period? The
Note that it might seem that I suggest that these ionization energies are a result of the position of the element in the Periodic Table. It is more correct to say that the modern Periodic Table reflects the physical and chemical properties of the individual elements. Metals tend to be electron-rich materials, that tend to be OXIDIZED, i.e. lose electrons, and their position on the Periodic Table (to the left as we face it!), reflects this. On the other hand, non-metals, say fluorine, and oxygen, tend to be reducing, i.e. they tend to accept electrons. Fluorine, in particular is the most oxidizing element on the Periodic Table:

