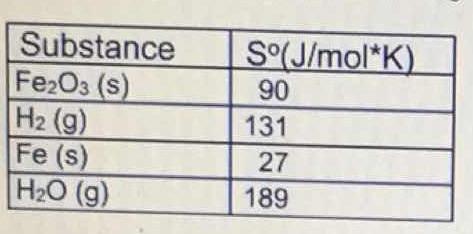
What is the value for #DeltaS^o"_(reaction)# for the following reaction, given the standard entropy values? #Fe_2O_3(s) + 3H_2(g) -> 2Fe(s) + 3H_O(g)#
1 Answer
Jun 28, 2017
Explanation:
For........
Has entropy increased or decreased?

