Is pressure an extensive or intensive property?
1 Answer
It must be intensive. It wouldn't make sense if a container on earth at so-called
We can see this from the definition of pressure:
#vecP = vecF/A# where
#vecP# ,#vecF# , and#A# are pressure, force, and surface area, respectively.
If the surface area over which the force acts is increased, the force is distributed over a wider range, so in order to keep the pressure the same, the force per unit area has to increase in magnitude. This occurs over time until the space is equilibrated.
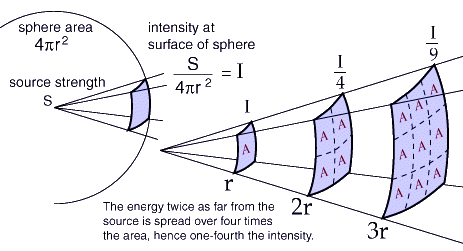
Having a ratio of two changing extensive properties (
And if we further imagine a room full of gas particles, if I asked you, "what is the pressure in this tiny region four feet above the ground here", you should be able to tell me, "it is the same as it is behind you, above you, and all around you".
That is, pressure in an equilibrated room of identical gas particles is uniform all throughout the room.
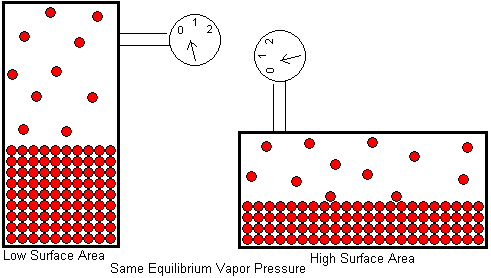
The force per unit area then must be the same throughout the room, so that no matter where you point, the force each gas particle exerts on the walls is identical to those of other gas particles (of the same identity).
If pressure were not intensive, we could not say that...

