Why is the heat of hydrogenation of benzene less than expected?
1 Answer
The heat of hydrogenation is less than expected because benzene is stabilized by resonance.
Explanation:
Heats of hydrogenation
Cyclohexene has one double bond, and its heat of hydrogenation is -120 kJ/mol.
Benzene has three double bonds, so we might expect its heat of hydrogenation to be -360 kJ/mol.

However, its measured heat of hydrogenation is only -208 kJ/mol.
Benzene is more stable than expected by 152 kJ/mol. This difference is called its resonance energy.
Resonance

We can write two Lewis structures for benzene, differing only in the positions of the electrons.
Whenever we can do this, the correct structure is neither of the two. It is a resonance hybrid of them both.
Such a structure is more stable than either of the contributors, and the extra stabilization is called the resonance energy of the compound.
The orbital explanation
We can understand this in terms of the orbitals involved.
The carbon atoms in benzene are
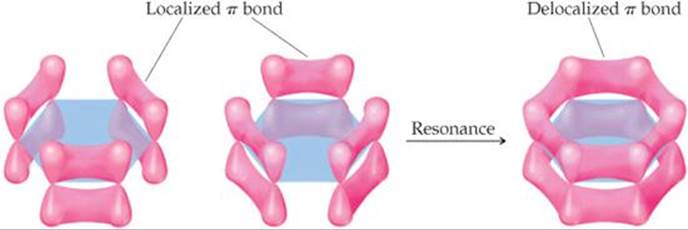
All six
Whenever electrons can spread out or "delocalize", they are at lower energy levels.
The overlap may appear to be small in the above diagram.
(Adapted from Physics Stack Exchange)
However, the computer-drawn picture above shows that

