What are #"f-elements"#?
1 Answer
Do you speak of the
Explanation:
The appearance of the lanthanides, and actinides in separate rows (i.e. Periods) on the Periodic Table, that are distinct from the transition metals is probably more a matter of formatting practicality. They would occur as rows, as Periods after lanthanum, and radium, BEFORE hafnium, and rutherfordium, but this would lead to a VERY wide Table.
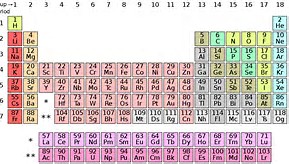
If you are following me, they would ideally appear at the asterisk, and the double asterisk, i.e. the gaps in the Table. But such a representation would give a Table that would be hard to fit on even on a landscape page.
Both groups show the filling of

