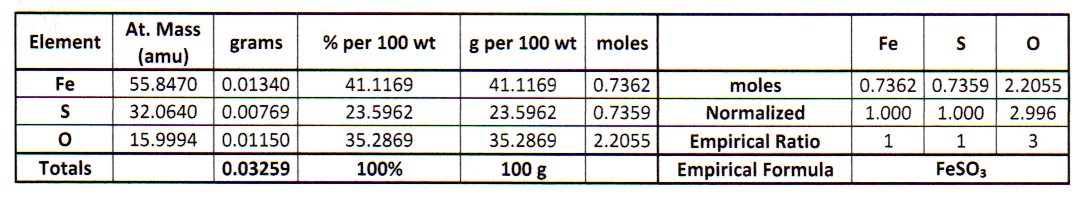
The empirical formula is the simplest whole number molar ratio of the elements in the compound.
We must convert the masses of #"Fe"#, #"S"#, and #"O"# to moles and then find the ratio.
#"Moles of Fe" = 0.0134 color(red)(cancel(color(black)("g Fe"))) × "1 mol Fe"/(55.84 color(red)(cancel(color(black)("g Fe")))) = 2.400 × 10^"-4"color(white)(l) "mol Fe"#
#"Moles of S" = "0.007 69" color(red)(cancel(color(black)("g S"))) × "1 mol S"/(32.06 color(red)(cancel(color(black)("g S")))) = 2.399 × 10^"-4"color(white)(l)"mol S"#
#"Moles of O" = 0.0115 color(red)(cancel(color(black)("g O"))) × "1 mol O"/(16.00 color(red)(cancel(color(black)("g O")))) = 7.188 × 10^"-4"color(white)(l) "mol O"#
From this point on, I like to summarize the calculations in a table.
#bb("Element"color(white)(Ag) "Mass/g"color(white)(Xm) "Moles"color(white)(Xmm) "Ratio"color(white)(m)color(white)(l)"Integers")#
#color(white)(m)"Fe" color(white)(XXXml)0.0134 color(white)(Xml)2.400 × 10^"-4" color(white)(Xll)1.000color(white)(mmmll)1#
#color(white)(m)"S" color(white)(XXXXm)0.00769 color(white)(mll)2.399 × 10^"-4" color(white)(Xll)1 color(white)(mmmmmll)1#
#color(white)(m)"O" color(white)(XXXXll)0.0115 color(white)(mml)7.188 × 10^"-4" color(white)(Xll)2.996 color(white)(mmmll)3#
The molar ratios are #"Fe:S:O = 1:1:3"#.
The empirical formula is #"FeSO"_3#.
Here is a video that illustrates how to determine an empirical formula.