Why is there a trend in first ionisation energies for elements in the same group?
1 Answer
In the same Group? You mean the trend for ionization energies in the same COLUMN of the Periodic Table.
Explanation:
We interrogate the ionization reaction......
To a first approximation, the Periodic trend is that ionization energies INCREASE across a row, a Period, of the Periodic Table, from left to right as we face the Table, and DECREASE down a Group, a column of the Periodic Table.
So why?
Incomplete electronic shells shield the nuclear charge VERY ineffectively. As protons are added to the nuclear charge across the Period, nuclear charge WINS over shielding by other electrons, with the result that if becomes more difficult to remove a valence electron, and ionization energies increase. Once a valence shell of electrons is FILLED, shielding of the nuclear charge becomes reasonably effective, and the ionization energies tend to decrease.
Is the graph consistent with what I have argued? Is there periodic behaviour in the ionization energy?
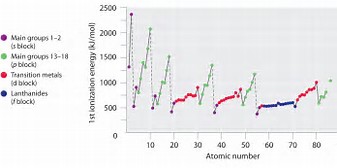
This effect is also manifested in the well-known decrease in atomic radii across the Period from left to right......How so?

