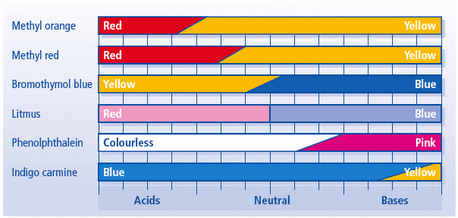
A solution of sodium hydroxide has a concentration of (2.1 x 10^-1)mol/L. What colour does the solution turn when a few drops of indigo carmine are added?
1 Answer
Jul 2, 2017
The solution will be will be yellow.
Explanation:
What is the pH?
What colour is the indicator?
Indigo carmine is blue at pH 11.4 and yellow at pH 13.0.
Since pH > 13.0, the indicator will be

