And for the molecular and electronic geometries we invoke simple VESPER.
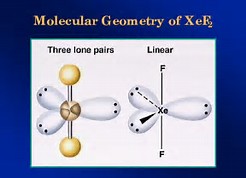
For #XeF_2# the electronic geometry is #"trigonal bipyramidal"#; the molecular geometry is #"LINEAR"#....
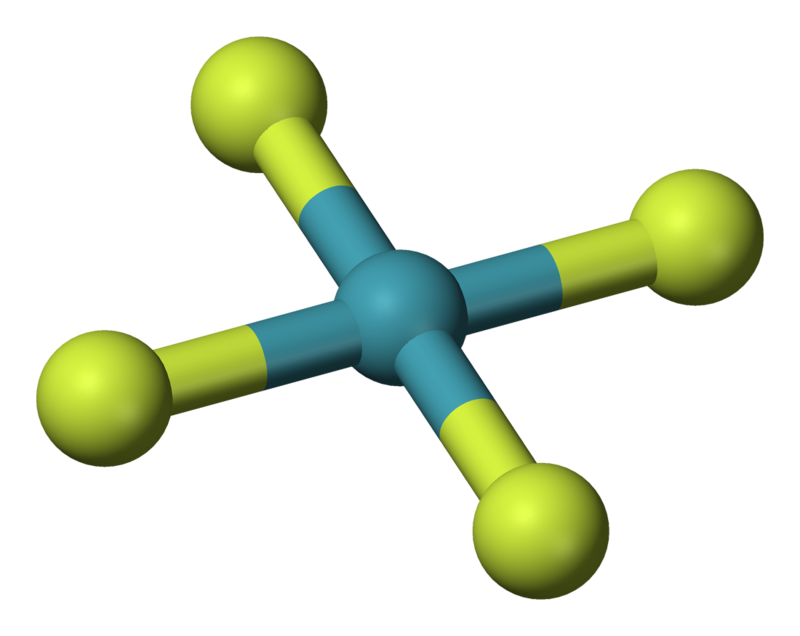
For #XeF_4#, we have #8+4xx7=36#, #"18 electron pairs"#. the electronic geometry is #"octahedral"#; the molecular geometry is #"SQUARE PLANAR"#....
For #XeF_6#, we got #8+6xx7=50, "25 electron pairs......"#; the central xenon is associated with 6 bonding, and 1 non-bonding pairs of electrons; a conformationally mobile, mono-capped octahedron structure is indicated with respect to the electronic distribution. See here for structure.



