What occurs when magnesium nitrate is mixed with potassium sulfate in aqueous solution?
2 Answers
In aqueous solution, we write
Explanation:
Neither the nitrate nor the sulfate salts are particularly insoluble......we would have an aqueous solution of all the ions......
ALL nitrates are soluble, and most SULFATES are soluble (except for those of
What would occur if I mixed
There will be no reaction.
Explanation:
Is the following reaction possible?
In order for this reaction to occur, one of the products must be an insoluble precipitate, an insoluble gas, or water. We can see that no water is produced and no insoluble gas is produced. So now we need to see if either of the products is an insoluble precipitate.
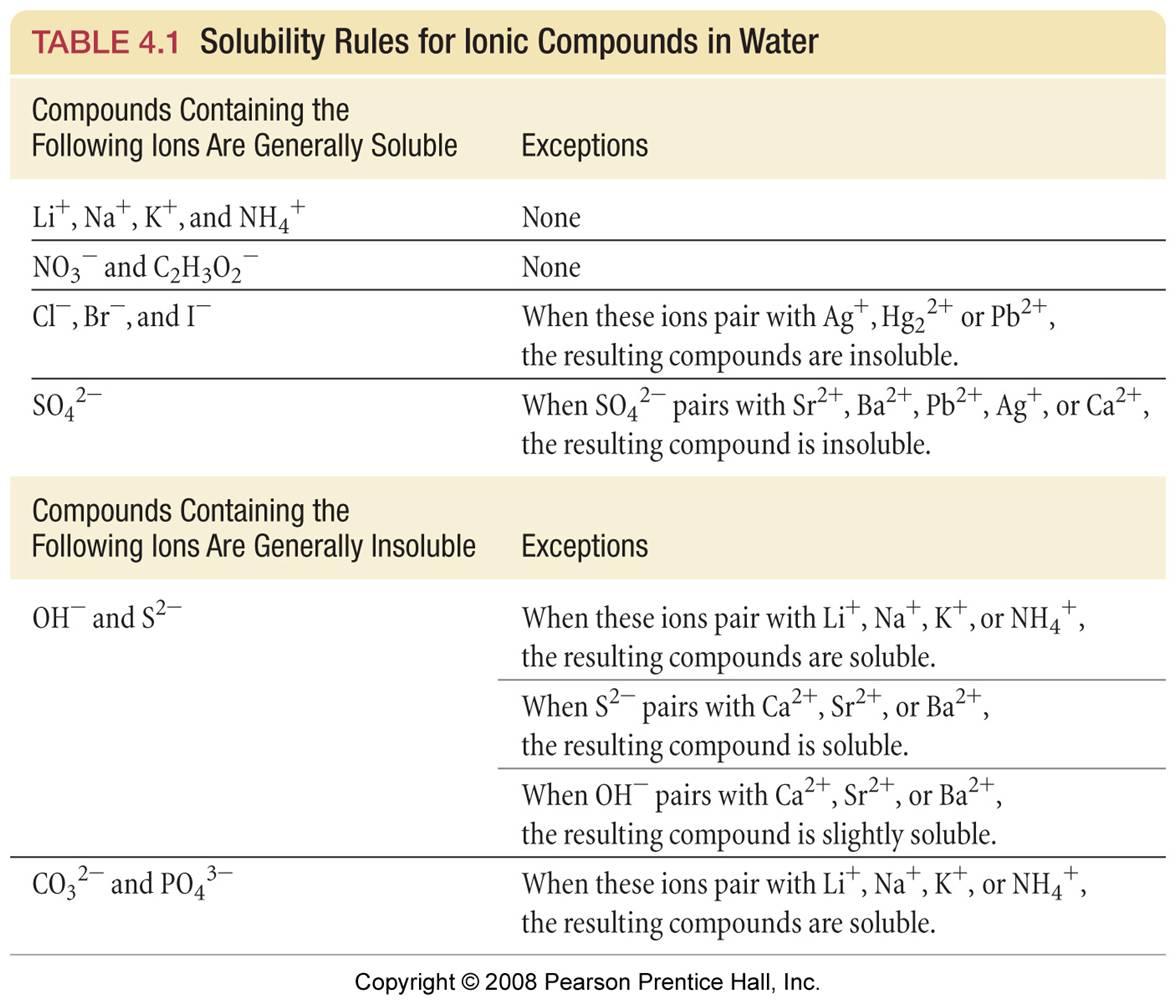
To determine if any of the products is an insoluble precipitate, we can consult a table of solubility rules.
According to the Solubility Table below,
In order to indicate that there is no reaction, you show that the reactants yield no reaction.