Do solids and liquids exert a vapour pressure?
1 Answer
Jul 8, 2017
I am not entirely clear as to what you are asking........
Explanation:
Liquids (and some solids) do exert an
The extent of the equilibrium is a function of temperature, and at the liquid's
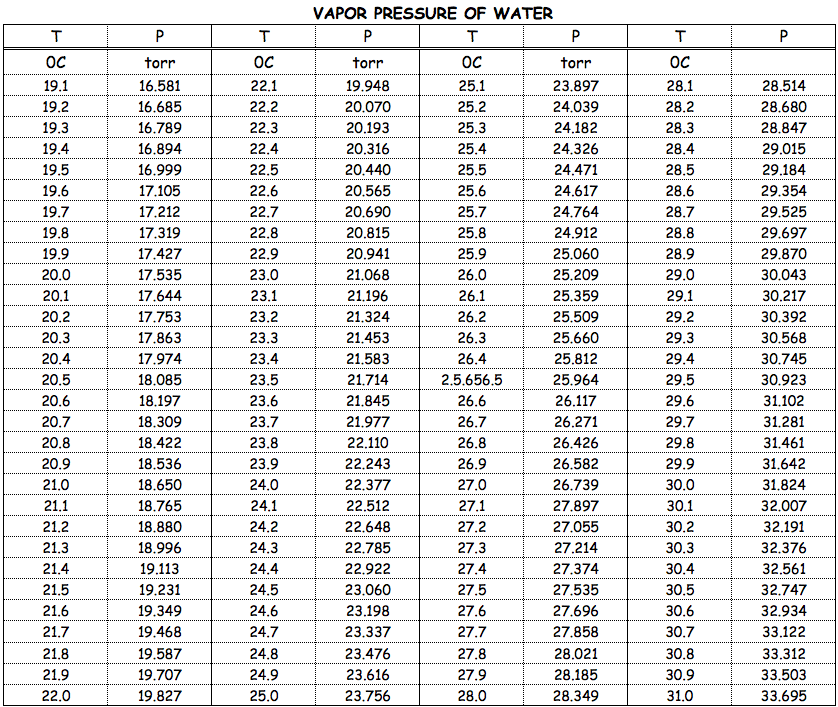
Sometimes, the vapour pressures are reported in

