Question #66091
1 Answer
Here's what I got.
Explanation:
Two things to keep in mind here
- the definition of a mole is given by Avogadro's constant
- we use subscripts to show how many atoms of a given element are present in one molecule or formula unit of a compound
So, you should know that in order to have exactly
This implies that in order to have
You can thus say that your sample contains
#color(blue)(2) color(red)(cancel(color(black)("moles C"_3"H"_8))) * overbrace((6.022 * 10^(23)color(white)(.)"molecules C"_3"H"_8)/(1color(red)(cancel(color(black)("mole C"_3"H"_8)))))^(color(purple)("Avogadro's constant"))#
# = color(darkgeeen)(ul(color(black)(1.2 * 10^(24)color(white)(.)"molecules C"_3"H"_8)))#
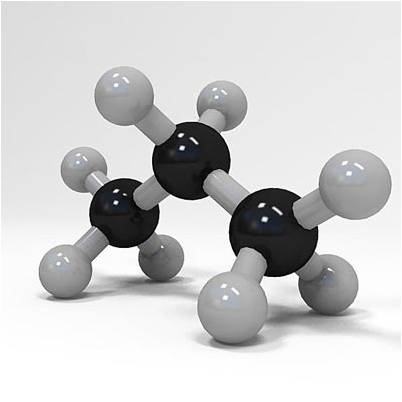
Now, a molecule of propane contains
- three atoms of carbon,
#3 xx "C"# - eight atoms of hydrogen,
#8 xx "H"#
You know that this is the case because of the two subscripts used in the chemical formula of propane.
#"C"_3"H"_8 implies {(3 xx "C"), (8 xx "H") :}#
You can thus say that your sample contains
#1.2 * 10^(24) color(red)(cancel(color(black)("molecules C"_3"H"_8))) * "3 atoms C"/(1color(red)(cancel(color(black)("molecule C"_3"H"_8))) )#
# = color(darkgreen)(ul(color(black)(3.6 * 10^(24)color(white)(.)"atoms C")))#
and
#1.2 * 10^(24) color(red)(cancel(color(black)("molecules C"_3"H"_8))) * "8 atoms H"/(1color(red)(cancel(color(black)("molecule C"_3"H"_8))) )#
# = color(darkgreen)(ul(color(black)(9.6 * 10^(24)color(white)(.)"atoms H")))#
I'll leave the answers rounded to two sig figs, but don't forget that you only have one significant figure for the number of moles of propane.

