Question #f4e10
1 Answer
Jul 25, 2017
There is a 'general' increase along a period, but a decreaee down periods.
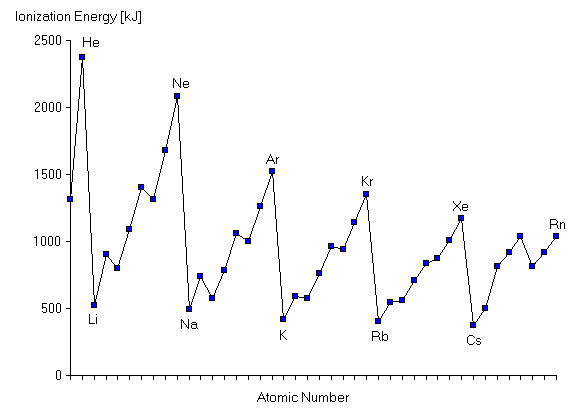
The increase along a period is caused by increasing nuclear charge, which will pull the electrons further in. The occaisonally drops occur when electrons enter a new orbital, this is because the new orbitals is further away from the njcleus, and so is attacted less towards it.
This is also the reason why it decreases down periods, the outer electron is further away and is therefore pulled less tightly by the nuclear charge, also there are many more electrons between the nucleus and outer electron, as the number pf eleftrons and orbitals increases, the shielding effect increases.

