Question #b33d8
2 Answers
I can't give you a "correct" answer with the information given, but I can show you how to solve it, which I did.
Explanation:
This is a really annoying unit conversion problem before we can even solve it, hold on:
You usually need the volume of one or the other solution in order to accurately solve this problem, but I will assume we are trying to neutralize
This appears to be a bit unreasonable, so perhaps in your problem the amount of citric acid needing to be neutralized is larger.
The required volume is
Explanation:
Step 1. Write the balanced equation for the reaction
or
Citric acid is a tribasic acid. Its titration curve will look something like the one below.
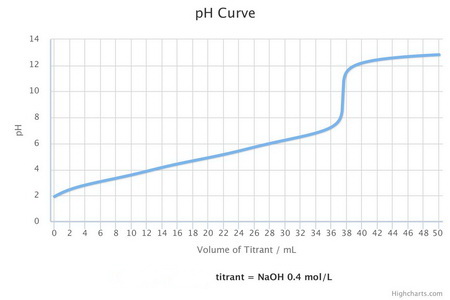 Titration curve
Titration curve
Step 2. Calculate the moles of
Step 3. Calculate the volume of
Note: The answer can have only one significant figure, because that is all you gave for the concentration of the
However, I calculated the answer to two significant figures.


