What is the ionization energy of water in kJ/mol? Is it endothermic or exothermic reaction?
2 Answers
The ionization of water is the energy associated with the process....
Explanation:
Now certainly this process is endothermic......
The autoprotolysis of water is represented by the equation......
, and again given,
As usual, I assume gas phase because it's ionization energy and we conventionally define ionization energy for gas phase particles...
#"H"_2"O"(g) + Delta -> "H"_2"O"^(+)(g) + e^(-)#
Fortunately, this process is well-known, and its enthalpy is:
#color(blue)(DeltaH_(IE) = "12.621 eV")# (It is interestingly easier to ionize water than
#"N"# atom, whose ionization energy is#"14.53 eV"# .)
I'll leave it as an exercise for you to convert to
#1.602 xx 10^(-19) "J" = "1 eV"# #"1000 J"# #=# #"1 kJ"# #"1 mol e"^(-) = 6.022 xx 10^(23) "electrons"#
and you should get approximately
#ul"1217.61 kJ/mol"# . Is positive enthalpy endothermic or exothermic? Do you think it requires energy input to remove an electron? (Try any similar exercise, like removing stems from cherries, caps from pens, etc., then decide.)
See the following MO diagram:
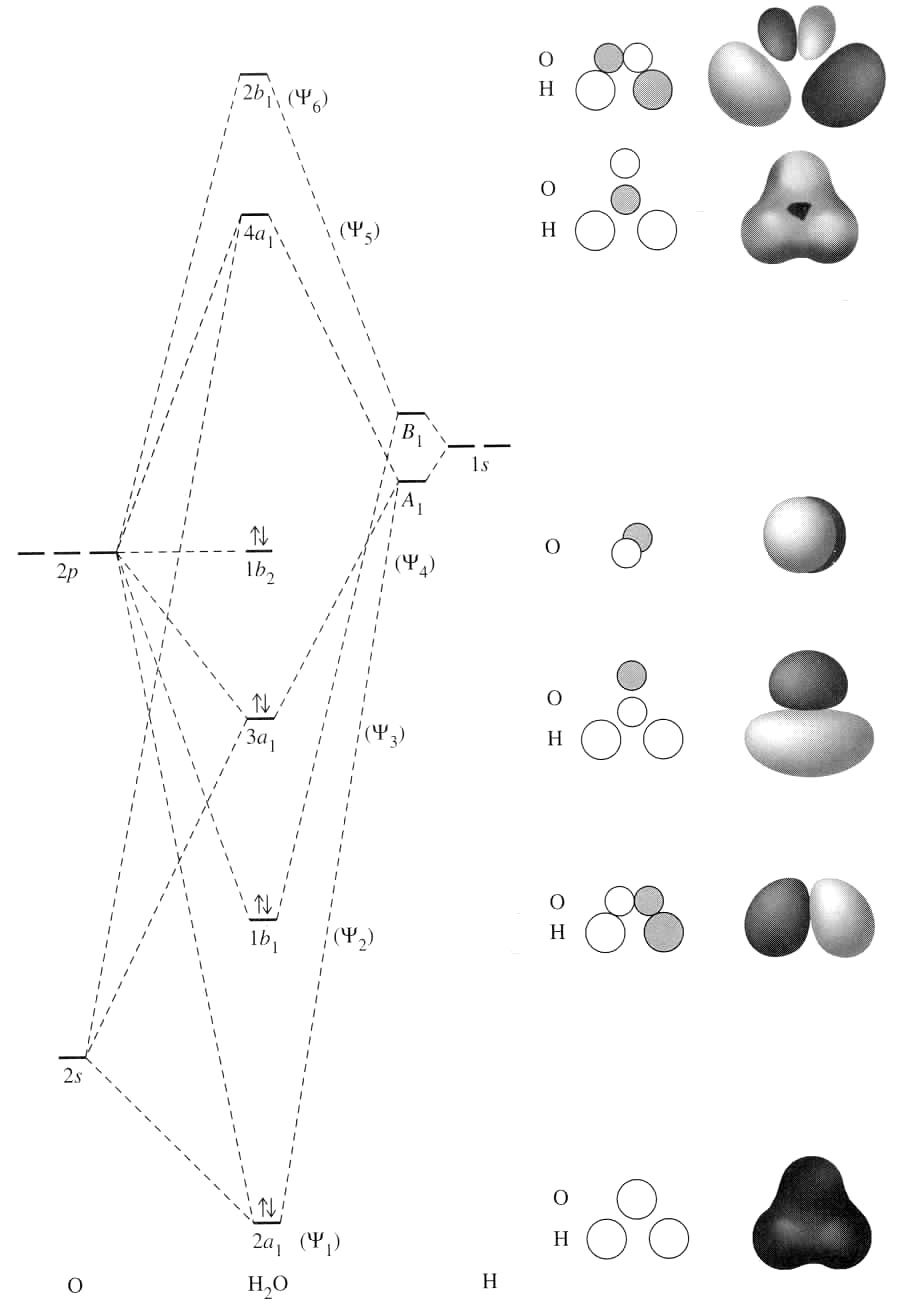
The ionization of water involves removal of the electron from its nonbonding
The oxygen then holds the formal charge of


