Are hydronium ions contributed to a solution by an acid or a base?
1 Answer
Brønsted-Lowry and Arrhenius *acids* introduce
Explanation:
In the Arrhenius acid definition, the acid introduces
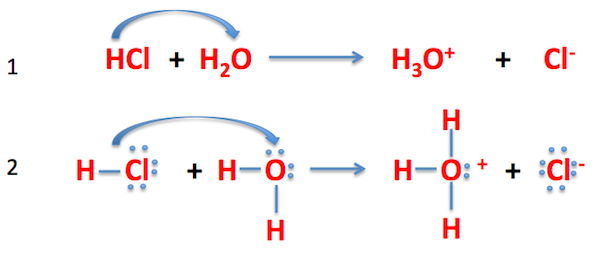
Here,
In the Brønsted-Lowry acid definition, the acid donates an

In this image,
Acids in the Arrhenius and Brønsted-Lowry definitions are quite similar; essentially, both definitions require for the acid to dissociate into
