Question #346a8
2 Answers
Explanation:
Trans butene looks like this
1) When the Br2 molecules passes near the pi bond of the C1-C2
bond the electron of the Br2 molecules get repelled and the Br
atom closer to the pi bond has less electron density and
acquires a positive charge while the other one which has
greater electron density acquires a negative charge.
The C1 would draw closer to the Br+ ion which would draw all
the electron density from the pi bond and C2 would be electron
deficient and would acquire a positive charge .When the Br+ ion
gets close enough it would bond with the C2 but it still remains
positive because the electron density from the pi bond has
been already drawn towards the C1.The C2 acts as an
electrophile in the next step.
2.) But what happens to the negative halogen ion? Yes it gets
attracted by the C2 which acts as an electrophile and it bonds to
the C2 from down position and the earlier bond of
Only one compound is formed
X = 1
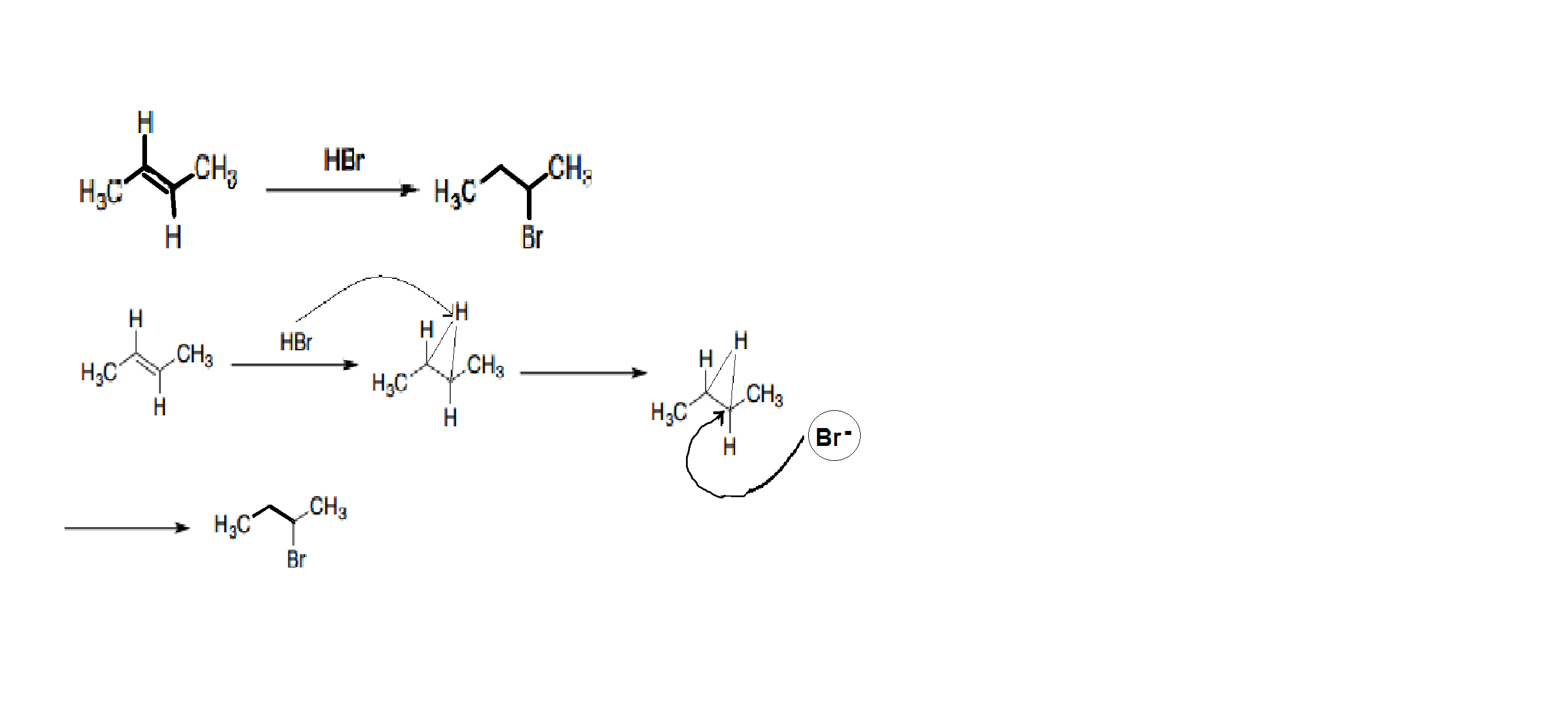
The next reaction is also the same but here instead of the one Br atom being the cation the Hydrogen is the cation here. And the conjugate base is the anion or the nucleophile.
And only one product is formed so
Y = 1
Explanation:
Reaction with
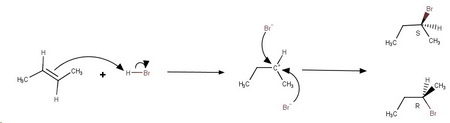
The reaction proceeds through a cyclic, chiral bromonium ion.
The bromide ion can then add to either carbon in the three-membered ring.

The two products are (
However, each of these has an internal mirror plane, so they are just different views of the same meso compound.
Since only one compound is formed as the product,
Reaction with
In the mechanism of this reaction, a proton attaches to one f the alkene carbons, generating a planar, achiral carbocation at the other carbon.
The bromide ion can attack this carbocation from either side to form two different products: (
Since two compounds are formed as the products,
If we report the answer as


