What is the formula for dinitrogen tetroxide?
1 Answer
Explanation:
We're asked to find the molecular formula of dinitrogen tetroxide.
This is a compound of nitrogen and oxygen (two nonmetals), so we speculate that it is a covalent compound (the prefixes also indicate it is a covalent compound).
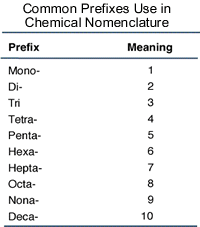
Nomenclature of chemical compounds is a fundamental aspect of chemistry, and for binary (two-element) covalent compounds like dinitrogen tetroxide, what we do is examine the prefixes:
The prefix in front of nitrogen is di-, so according to the above image, we know that there are
The prefix in front of the oxide (oxygen) species is tetra- (the "a" is left off if oxygen is the second element), so there are
We put the numbers of each element as subscripts of the element's symbol, so the formula is
#ulbar(|stackrel(" ")(" "color(red)("N"_2)color(blue)("O"_4)" ")|)#