What is the wavelength of light that causes an electronic transition for #"Li"^(2+)# if #n_f - n_i = 2# and #n_i + n_f = 4#?
1 Answer
I got
Well, we first dissect the minor puzzle here... Your system of equations is:
#n_f - n_i = 2#
#n_i + n_f = 4# where:
#n_f# is your destination energy level in a hydrogen-like atom.#n_i# is the energy level from which the electron started transitioning in that hydrogen-like atom.
By simple substitution,
#n_f = 2 + n_i#
#=> n_i + 2 + n_i = 4#
#=> ul(n_i = 1)#
#=> ul(n_f = 3)#
Indeed,
Now that we know the electronic transition is
#bb(DeltaE = -Z^2 cdot "13.61 eV"(1/n_f^2 - 1/n_i^2))# where:
#DeltaE# is the change in energy due to the transition.#-"13.61 eV"# is the ground-state energy of hydrogen atom. The magnitude,#R_H ~~ "13.61 eV"# , is also called the Rydberg constant.#Z# is the atomic number.
The wavelength
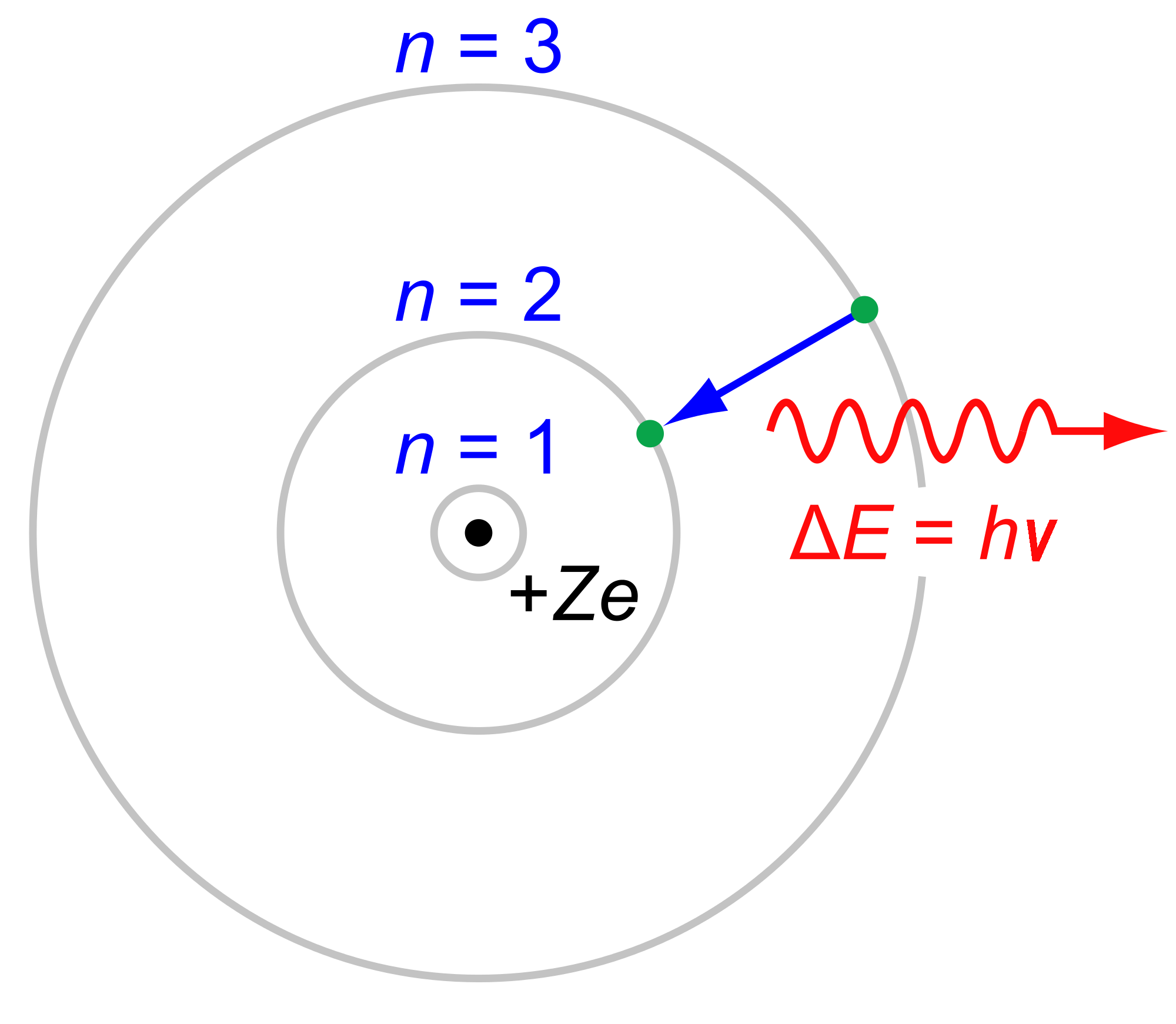
#bb(DeltaE = E_"photon" = hnu = (hc)/(lambda))# where:
#h = 6.626 xx 10^(-34) "J"cdot"s"# is Planck's constant.#c = 2.998 xx 10^(8) "m/s"# is the speed of light.#nu# is the frequency of the photon in#"s"^(-1)# .
And so, the Rydberg equation for wavelength is:
#bb((DeltaE)/(hc) = 1/lambda = -(Z^2 cdot "13.61 eV")/(hc)(1/n_f^2 - 1/n_i^2))#
Knowing that, the wavelength will be:
#lambda = [-(3^2 cdot 13.61 cancel"eV" xx (1.602 xx 10^(-19) "J")/(cancel"1 eV"))/((6.626 xx 10^(-34) "J"cdot"s" xx 2.998 xx 10^(8) "m/s"))(1/3^2 - 1/1^2)]^(-1)#
#=# #1.139 xx 10^(-8) "m"#
In nicer units, we then have:
#color(blue)(lambda) = 1.139 xx 10^(-8) cancel"m" xx (10^9 "nm")/(cancel"1 m")#
#=# #color(blue)(ul"11.39 nm")#
Just to be sure you get the right value, the energy you get, which is easier to calculate, would have been:
#color(blue)(DeltaE) = -Z^2 cdot "13.61 eV"(1/3^2 - 1/1^2)#
#= -3^2 cdot "13.61 eV"(1/9 - 1/1)#
#=# #color(blue)(ul"108.88 eV")#
And to check, we look on NIST. The energy levels for
These are in
#lambda_(ref) = 1/("877919.12077 "cancel("cm"^(-1))) xx (10^7 "nm")/(cancel"1 cm")#
#=# #"11.3906 nm"# #~~# #"11.39 nm"#
So, the wavelength we got is correct.

