On what factors does #"ionization energy"# depend?
1 Answer
On
Explanation:
And ionization energies clearly indicate a Periodic Trend that manifest the two given properties. Ionization energies INCREASE across the Period, a row, from left to right as we face the Periodic Table, and DECREASE down a Group, a column of the Table.
Now it is a fact that INCOMPLETE electronic shells shield the nuclear VERY INEFFECTIVELY. Consider the following diagram......
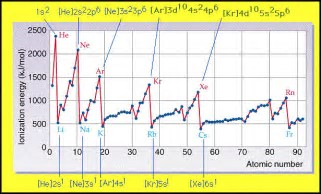
And clearly, the LARGEST ionization energy, (which represents the enthalpy change for the following reaction......
......occurs for the Noble Gases, which have the highest nuclear charge for a given electronic shell.
A full valence electronic shell, shields the nuclear charge fairly effectively, and thus the alkali metals, with a single valence electron, have demonstrably low ionization energies........

