What processes can be considered the chemical reverse of each other?
1 Answer
Check out the answer.
Explanation:
There is one chemical process in biochemistry that has a reversible process. If you remember glycolysis, it actually has a reversible process. This reversible process is called gluconeogenesis. These two processes are reversible in response to glucose concentration in our bodies. When our bodies need energy, glucose breaks down into pyruvate through glycolysis. When our bodies need glucose for storage, pyruvate turns back into glucose through gluconeogenesis. Here are the processes:
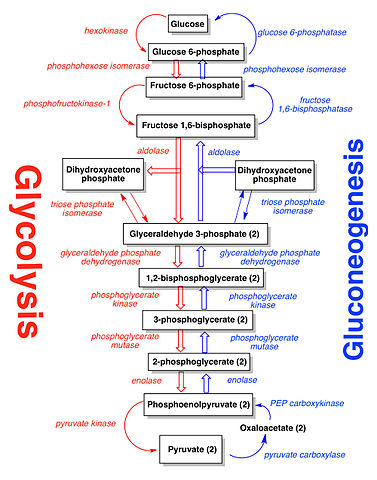
On a larger scale, photosynthesis and cellular respiration are "reversible" processes in terms of the chemical equations. Look at the following reaction. The forward is cellular respiration. The reverse is photosynthesis (energy can be ignored).
Weak acids and bases are chemically reversible. Here are their equations:
With this in mind, another biological function is the bicarbonate buffer system. This is a pH regulation system in our bodies. Normally, bicarbonate is much more present than carbonic acid. If there are any fluctuations in the pH in our bodies, this buffer system will correct for it. Since carbonic acid is not stable, it can break down into carbon dioxide and water. This is also reversible. Here are the two reactions as one:
Another process that is reversible is the Haber process. This is an artificial nitrogen fixation process that generates ammonia from nitrogen and hydrogen gases. Here is the chemical reaction:

