Question #efe36
1 Answer
D
Explanation:
Here is the reaction that a generic alkene goes through. This is called a hydroboration-oxidation reaction.
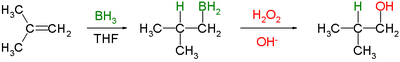
The hydroboration reaction is a stereospecific and regioselective reaction. It is stereospecific adds syn. This means that it adds to the same side. It is regioselective due to steric factors. Steric factors control regioselectivity more than electronic factors. The borane will add to the less substituted carbon.
This was discovered by H.C. Brown. Borane adds to the same side by forming a complex due to shifts in electron density. During the transition state, a hydrogen from borane is transferred to the neighboring carbon. The result of this part of the reaction is an alkylborane. This can be seen in top line of the following mechanism.
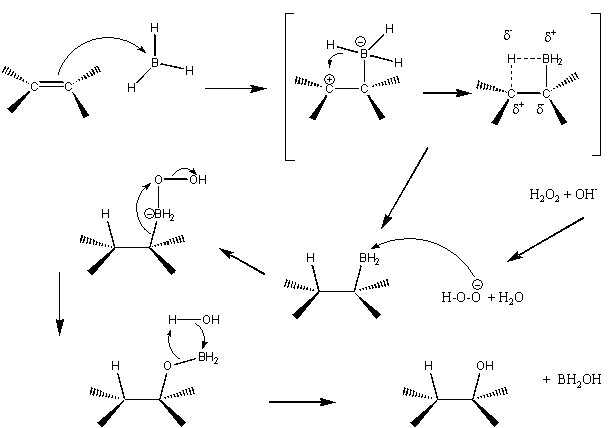
Next is the oxidation reaction. This consists of water, sodium hydroxide, and hydrogen peroxide. The sodium hydroxide is used to deprotonate the hydrogen peroxide so that it can react with the alkylborane. Once it reacts, water must remove the borane. This happens by a few things happening at once as seen in the bottom left hand of the second picture above. The result is an alcohol and boric acid.

