Question #dfec5
1 Answer
Aug 21, 2017
Of course you can, but it is not a reasonable structure.
Explanation:
It looks as if you are trying to generate a resonance contributor for the azide ion,
What's wrong with a structure like this:
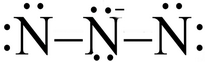
(Adapted from ResearchGate)
The two terminal nitrogen atoms do not have an octet!
You can draw three other structures in which all atoms have octets:

If it is possible to give all atoms an octet, a structure in which an atom does not have an octet is not a reasonable contributor.

