Carbonyl groups usually undergo stretching vibrations.
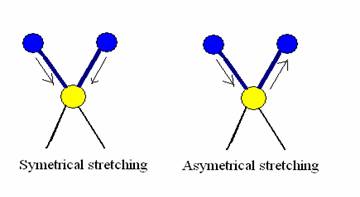
The #"C=O"# group is one of the most easily recognized peaks in an IR spectrum. The change in dipole moment is significant, making this an intense band, and there are few other groups that give rise to absorbances in the #1600-1850 "cm"^-1# range
Small rings and angle strain make this frequency even more intense.
For example in cyclo-butanonane
https://comptox.epa.gov/dashboard/dsstoxdb/show_image?source=61592
The C-CO-C angle is #90^@# which is less than the normal #120^@#
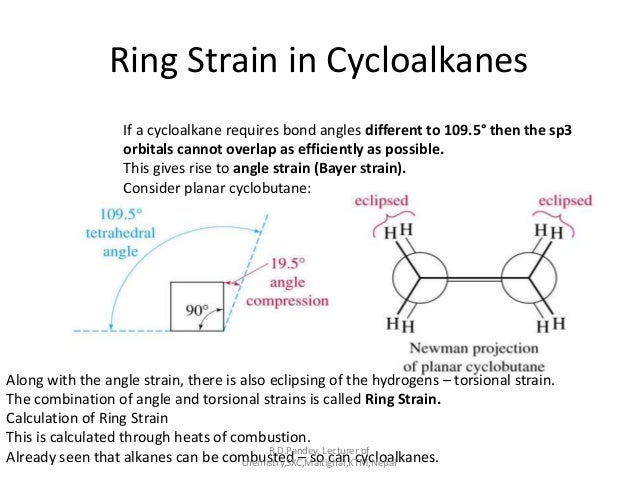
As the ring carbons shared #pi# orbitals with the carbon atom in the carbonyl group, when the bond angle is compressed the #pi# orbitals no longer strongly overlap.The s-character of the #"C=O"# increases which strengthens the bond.
The s-character is increasing the stability because in p orbitals you are moving the bond electrons to a higher energy level.
Therefore the order of energy of bonding orbitals
#"sp" < "sp"^2<"sp"^3#
This makes the frequency of #"C=O"# more by increase the bond energy.



