What is the mass of 1 mol of an element?
2 Answers
Aug 28, 2017
It is the molar mass of the element.
Explanation:
The molar mass of an element is its atomic weight (relative atomic mass) on the periodic table in grams/mole, or g/mol.
For example, the atomic weight of oxygen (O) on the periodic table is
Aug 28, 2017
It is the atomic weight in grams for the metric system.
Explanation:
The "mass" of an element is based on a comparison to an accepted standard mass, and may also be subject to the averaging of variations in normal isotopes of an element.
The "mol" is simply a number of units as derived by Avogadro to be
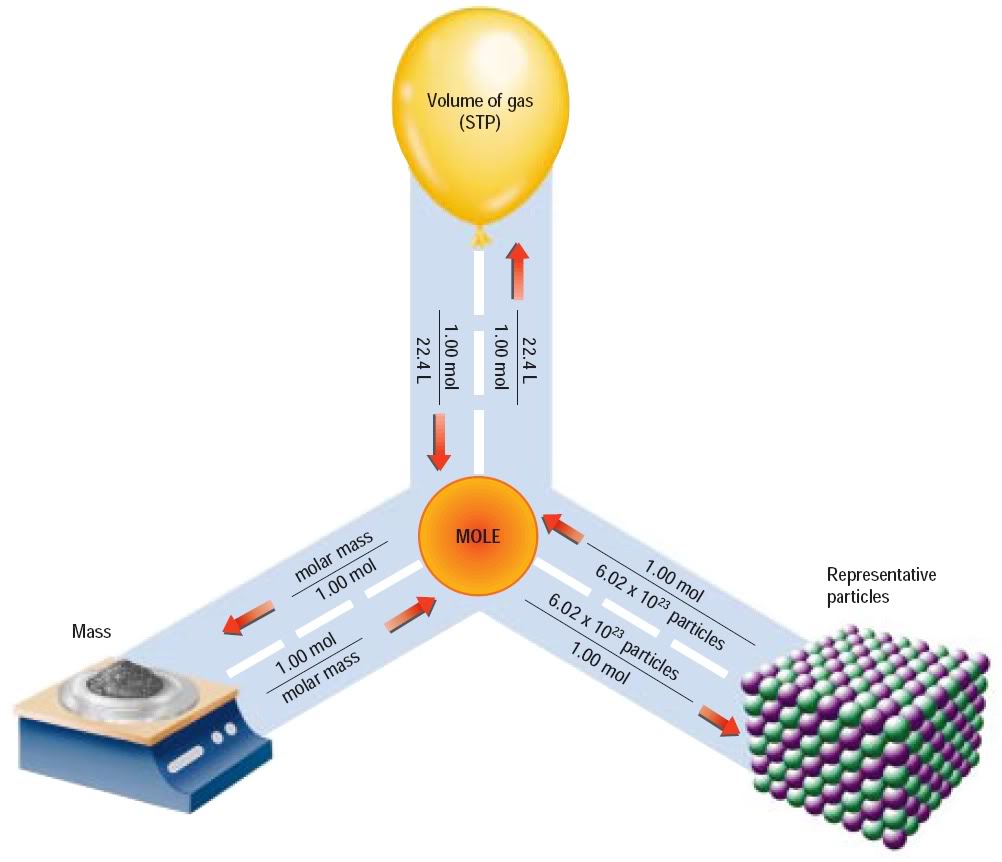
http://itschemistrytime.blogspot.com/2011_11_01_archive.html


