Which end of the phospholipid is attracted to water?
2 Answers
phospholipid is basically lipid with phosphate group
Explanation:
A phospholipid has two components:
- Phosphatidic acid
- Amino alcohol
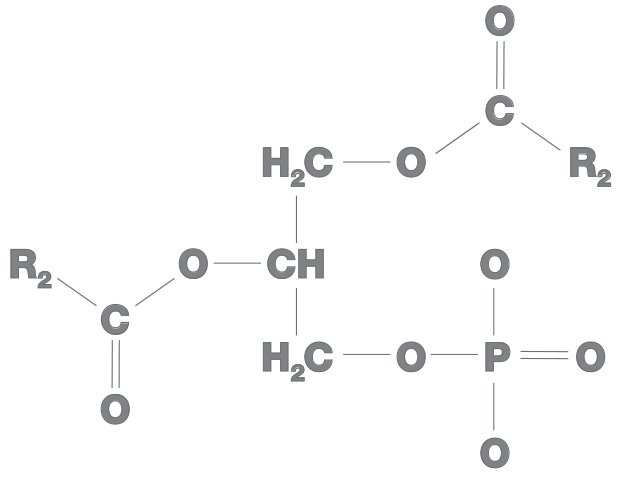
All phospholipids have phosphatidic acid as their backbone and an amino alcohol.Amino alcohol also is of three types:
- Coline
- Ethnaloamine
- Sphingosine
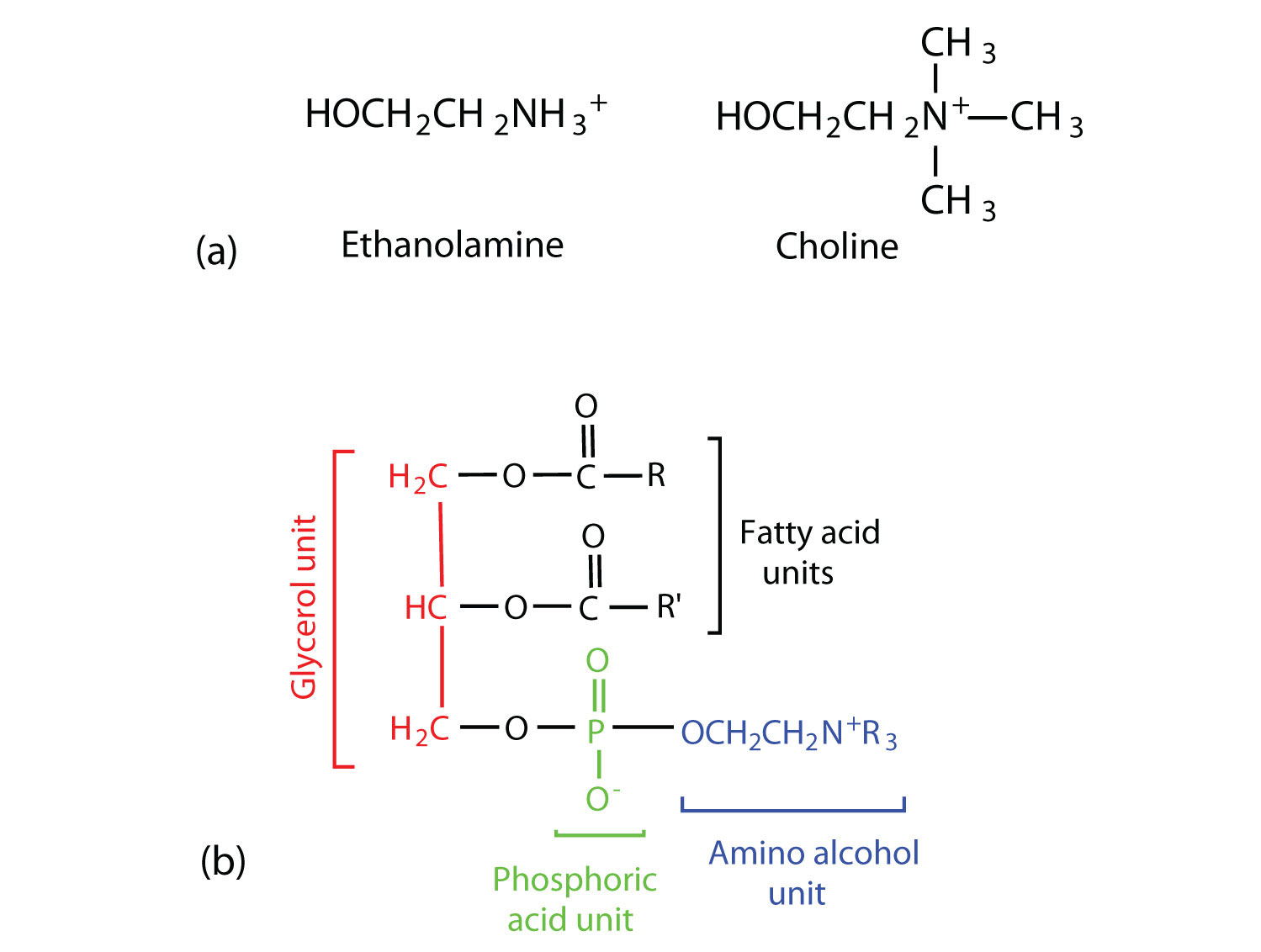
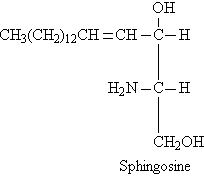
When coline binds with phosphatidic acid, it forms lesithin. When ethnaloamine binds with phosphatidic acid, it forms cephaline and when sphingosine binds with phosphatidic acid, it forms sphingolipid.
In all phospholipids, the phosphatidic acid is hydrophobic while amino alcohol is hydrophilic.
The end containing the phosphate group is attracted to water.
Explanation:
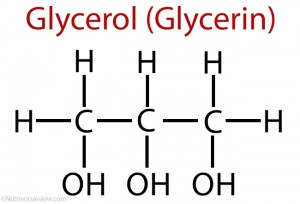
A phospholipid is a molecule that is formed by the condensation of:
- a glycerol molecule
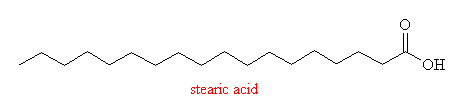
- two fatty acids
- a phosphate group
- an alcohol
The most common phospholipid in the human body is lecithin.
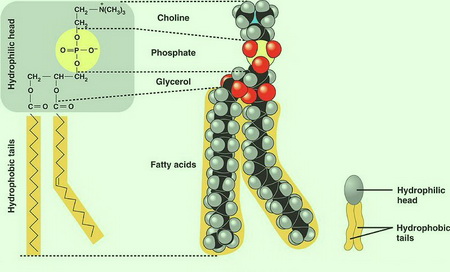
(From Seven Days per Week - blogger)
The components of a molecule of lecithin are
- choline

-
phosphoric acid,
#"H"_3"PO"_4# -
glycerol
- stearic acid
- oleic acid

The end containing the choline and the phosphate group is called the hydrophilic head of the molecule.
The choline contains a positive charge and the phosphate has a negative charge, so they are highly solvated by water molecules.


