Can someone explain to me the concept of entropy?
2 Answers
Probably, it might not be me tho....
Explanation:
Entropy is the statistical probability for disorder...... It is a fundamental property of any system, and can certainly be measured. The typical units of entropy are
So some, analogies......when we open a new deck of cards (and have you ever done this?), the suits are all in order, and the cards progress from ace to king......Shuffle the cards well, and you proceed from an highly ordered-state to a highly disordered state. The entropy, the randomness, has INCREASED.
When we deal with chemical systems, the opportunity for entropy is greatly magnified. And entropy can be summed appropriately for a chemical or physical system.
We often speak of
Standard tables of entropy exist to calculate
One way I would describe entropy, in the modern day definition, is...
the system's ease to which it disperses energy amongst its available energy states.
For example, as gases can easily attain high heat capacities due to their greater number of degrees of freedom (particularly in translational motions), they can store more energy in their available states, and thus have more entropy than solids and liquids.
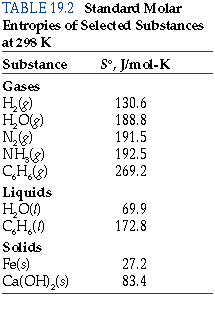
- This is seen quantitatively by examining the standard molar entropies
#S^@# in the back of your general chemistry textbook.
You should find that
#S^@# for gaseous substances is greater than for the respective liquid or solid phases.
- This is seen qualitatively in that gases can move around more freely than liquids and solids.
Since the energy level spacings between translational energy levels is much, much smaller than
#k_BT# at most any temperature, translational motion is the major contributor to the entropy of a system, and that is absolutely abundant in gaseous systems.
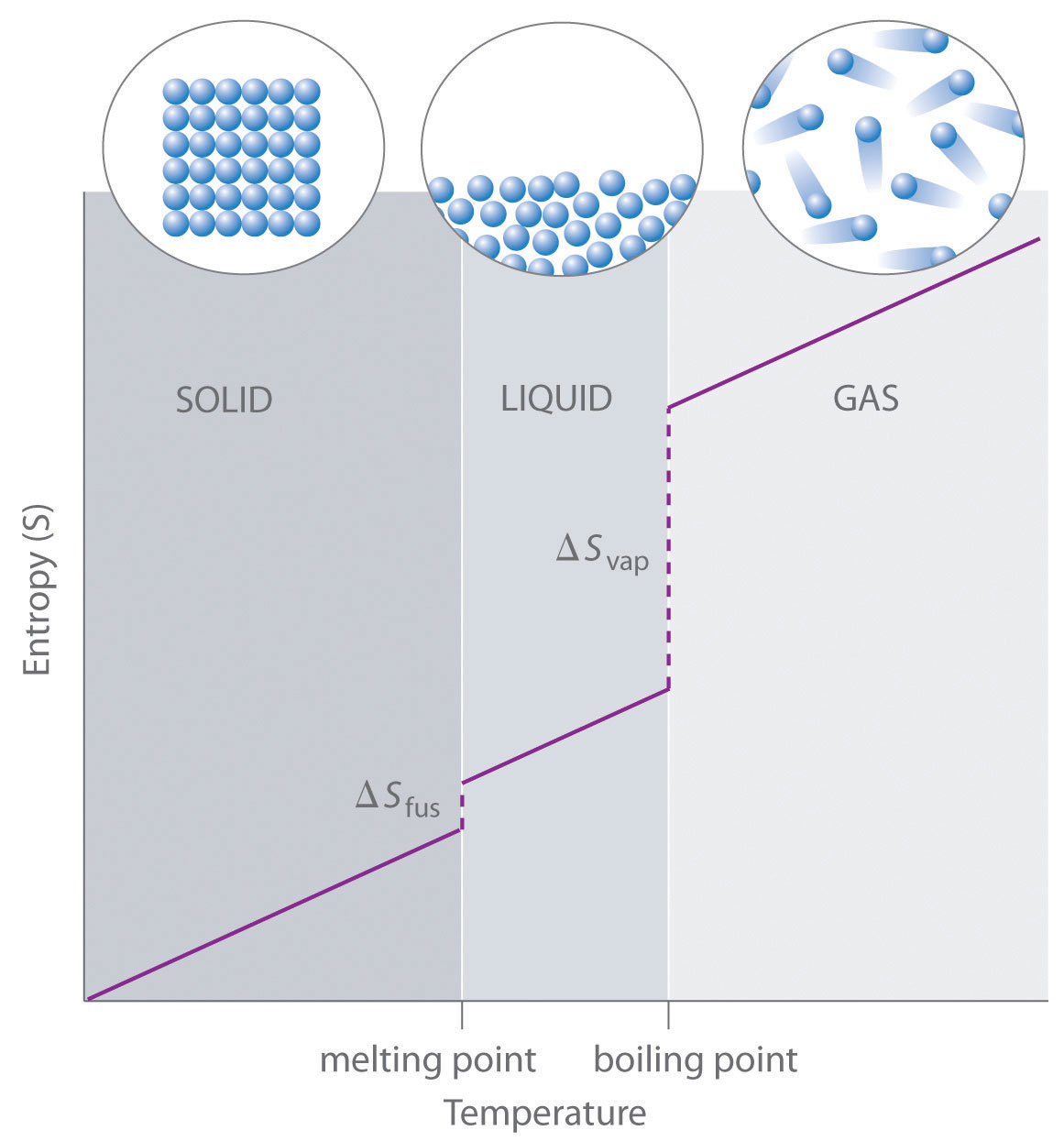
In short, entropy is proportional to the amount of motion in the system.


