Question #3a8f2
1 Answer
Sep 1, 2017
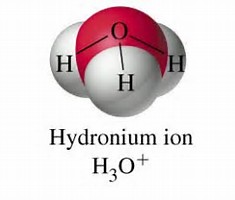
Well, as with water, the electron pairs are arranged in a tetrahedron....
Explanation:
And thus, we got
So the symmetry descends to trigonal pyramidal, i.e. analogous to ammonia: