Question #ce2b6
1 Answer
Sep 9, 2017
It is some what like this *
Explanation:
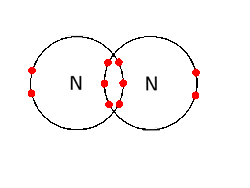 http://ibchem.com/IB/ibnotes/04.2.htm
http://ibchem.com/IB/ibnotes/04.2.htm
As nitrogen has its outer shell valence 5 it needs more 3 electrons to become stable therefore , it becomes stable by covalently bonding with another nitrogen atom . The outer shell becomes stable when both the nitrogen atoms shares 3 electrons with each other like done in the above diagram . The above diagram is dot and CROSS structure (as it is easy to understand I showed you this )
This diagram below is the electron dot diagram (lewis dot structure).
(
