Question #c0425
1 Answer
(i) The number of moles does not change, but the molar concentration decreases.
(ii) The number of moles decreases, but the molar concentration does not change.
Explanation:
(i) Dilution
(a) Moles of solute
When you add more solvent, the volume of the solution increases but the number of particles stays the same.
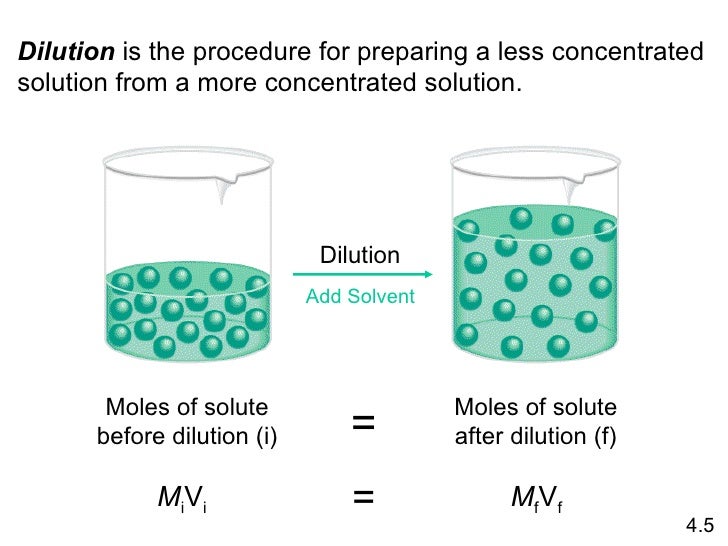
The number of moles of solute does not change.
(b) Molar concentration
The formula for molar concentration (
#color(blue)(barul|stackrel(" ")(c = "moles"/"litres")|)#
When you add more solvent, the volume increases but the number of particles stays the same.
Since "litres" is in the denominator, increasing the volume decreases the molar concentration.
(ii) Transferring some of the solution to another beaker
(a) Moles of solute
Let's say that you have 1 mol of solute in 1 L of solution, so
When you transfer
transferred
Only
∴ The number of moles decreases.
(b) Molar concentration
In the original beaker,
In the second beaker,
The molar concentration does not change.

