Question #e9eb6
1 Answer
Sep 19, 2017
Diborane is nonpolar because it is a symmetrical molecule.
Explanation:
The structure of diborane is
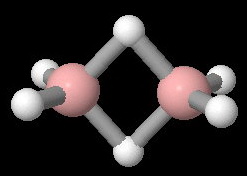
Two of the
Theoretical calculations show that the partial charges on each atom are:
#"B" color(white)(mmmmll)= "+2.12"# #"Bridging H" = color(white)(ll)"-0.71"# #"Terminal H" = color(white)(l) "-0.70"#
Thus, there are fairly large
However, the diborane molecule is perfectly symmetrical.
Every
Thus, all bond dipoles cancel and diborane is a nonpolar molecule.

