Question #13e75
1 Answer
Sep 24, 2017
Heat causes matter to change state.
Explanation:
Heat is a form of energy. It just so happens that this energy is what "excites" particles.
If a substance gains energy (AKA its temperature increases), then the particles' behaviour will reflect the energy gained in the form of phase changes.
This is because as the particles gain energy, they slowly break free from the attraction that binds them in the state arrangement.
As a result, the substance changes state to accommodate the change from solid -> liquid -> gas.
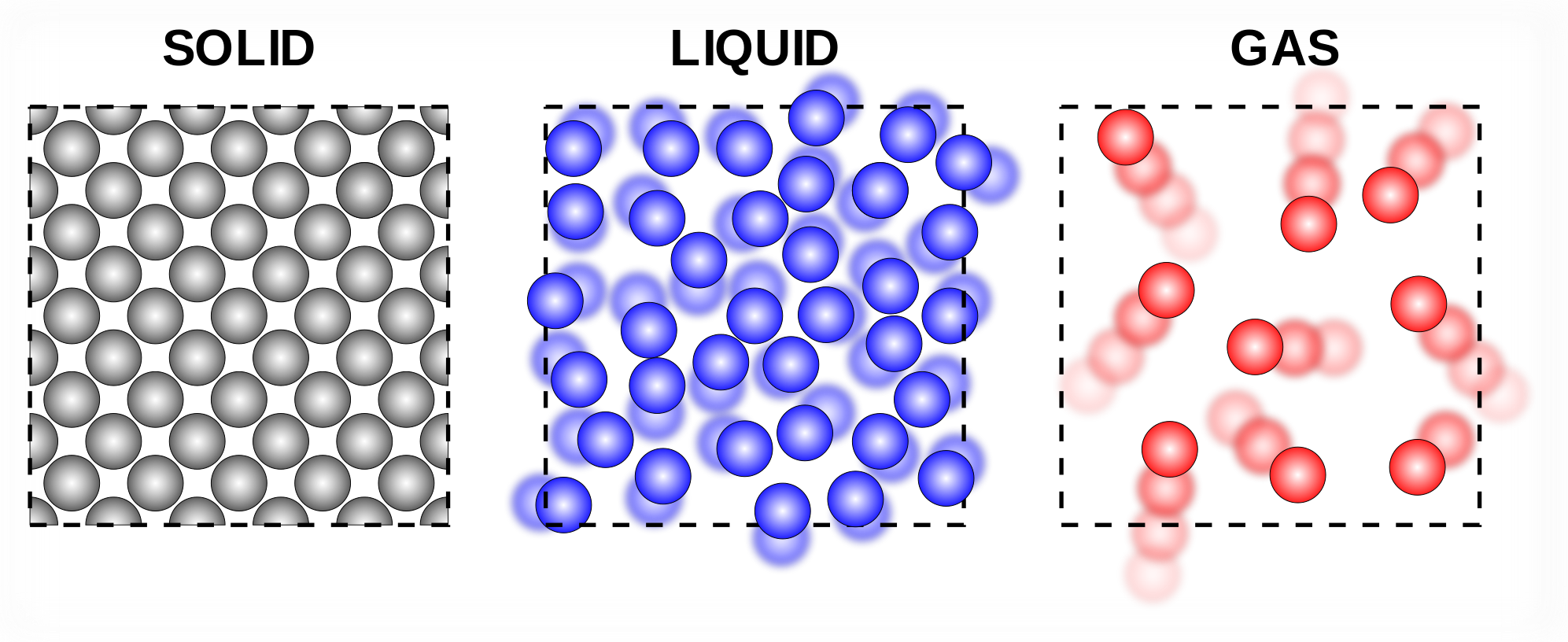
This is the same if substances lose energy (get colder), except it's the opposite way gas -> liquid -> solid.
Hope this helps :)

