What would a six coordinate metal-ligand complex structure look like?
2 Answers
The bonds are in an octahedral shape.
Explanation:
Let's take Sulphur hexaflouride (SF
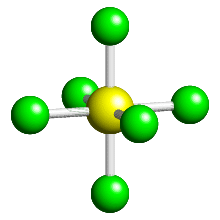
Since a complex is just molecules forming dative covalent bonds with a metal, the idea works the same way. Molecules will spread out evenly, for example in hexaaquairon(II) there are 6 water molecules dative covalently bonded to the Fe

Another example is EDTA, its a polydentate ligand that forms 6 dative covalent bonds. When it bonds to a metal ion, all 6 bonds are created, and are also spread out too.
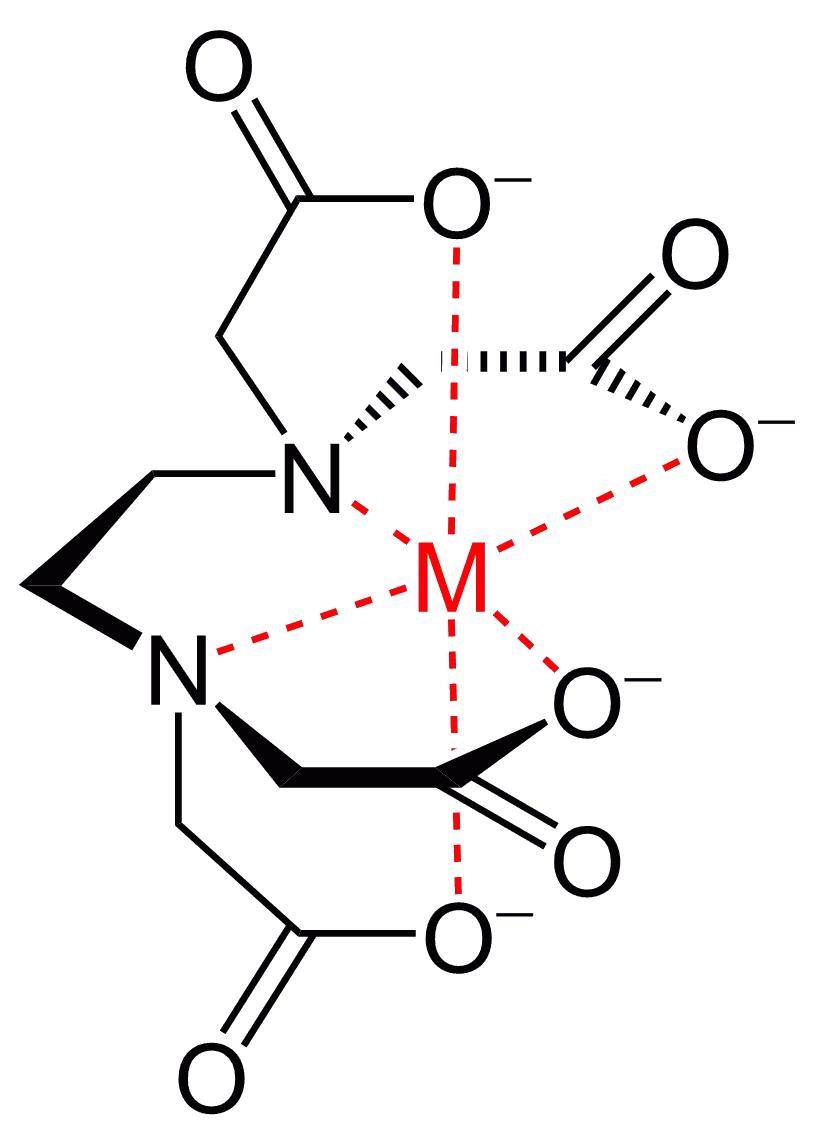
Most likely, octahedral.
There are exceptions, but for the most part, a simple metal-ligand complex is usually octahedral.
This just forms the most symmetric geometry, with equal bond angles for all adjacent ligands. It therefore minimizes bond-pair-bond-pair electron repulsions in most cases.
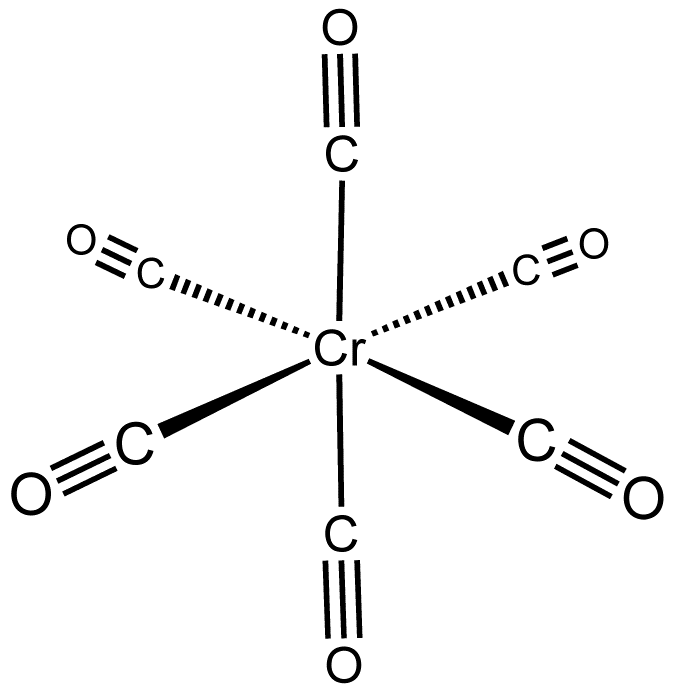
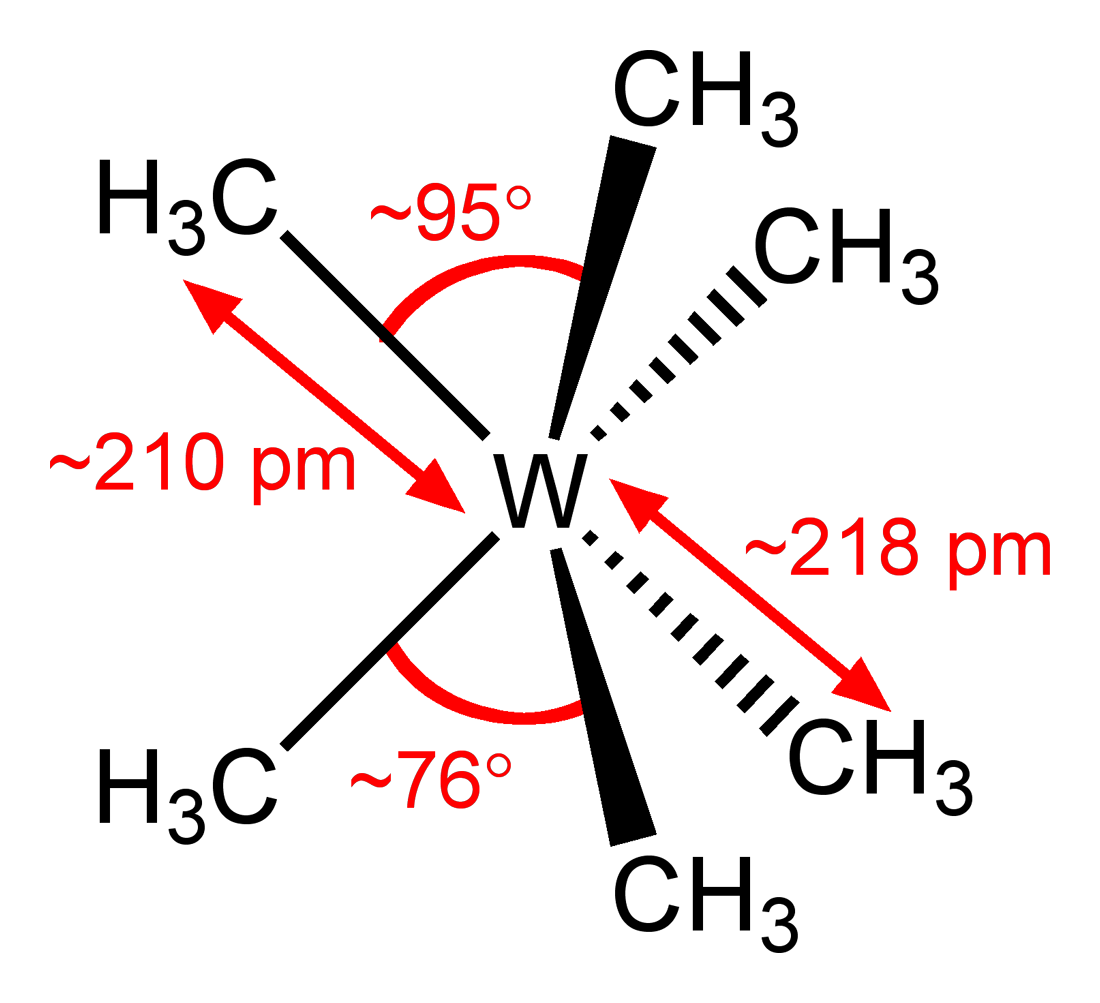
The main exception is trigonal prismatic:
This particular compound is air-sensitive, subliming at
It is thermodynamically stabilized due to tungsten's
Apparently, the trigonal prismatic shape is a result of a so-called second-order Jahn-Teller distortion.