How many bonds do atoms make?
1 Answer
The number of bonds an atom can form depends on that atom's valence electrons.
Explanation:
Short Answer: An Atom can go any distance to complete its octet!
LongAnswer:
Carbon the most common atom in organic compounds can form four bonds because it has 4 valence electrons. Therefore, needs four more electrons to complete the octet.
Nitrogen is needed to from proteins, DNA and RNA. It has 5 valence electrons. So it needs 3 more to fill up its octet. So it can form three, or at most four bonds.
Oxygen needed for Carbohydrates and Carbon Dioxide forms two bonds.
Hydrogen needed for Carbohydrates can form one bond.
Sulfur used in making some amino acids can form 2 , 4 or 6 bonds.
So, The number of bonds an atom can form depends on the atom or more appropriately its valence electrons. This is shown for every atom in this picture:-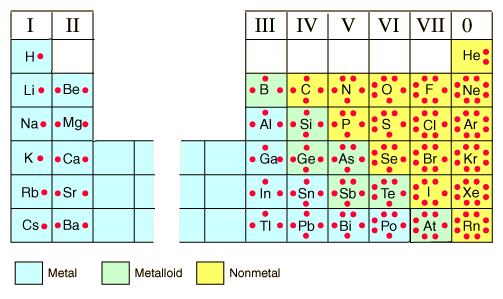 http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/lewis.php
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/lewis.php

