Are the bond angles in water, and carbon tetrachloride the same?
2 Answers
Only to a first approximation?
Explanation:
In
In water, the equivalent
Both molecules are formally
How would you describe the geometry of
Bond angles are different between
Explanation:
Both molecule have
Carbon tetrachloride(
In contrast, water(
Therefore, angle between lone pairs must be larger than
Bond angle between two
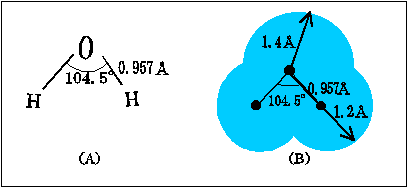
(The picture is cited from a Japanese site, "水の話 ~化学の鉄人小林映章が「水」を斬る!~ " http://www.con-pro.net/readings/water/doc0002.html)


