What would happen to cells in a solution if water is added?
1 Answer
It depends on the salinity of the solution.
Explanation:
If it is a normal saline solution (NSS), then nothing will happen to it since NSS mimics the composition of the human body. NSS is an example of an isotonic solution, wherein the amount of solute is equal inside and outside of the cell.
If the solution was hypertonic (concentrated with salt), the the cell would shrink as water from inside will pass through the cell wall to go outside, because of the higher concentration of solute (osmosis).
If the solution was hypotonic, then the cell will bulge as water then goes inside the cell wall where there is a higher concentration of solute.
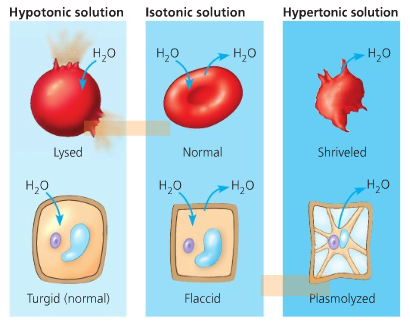 https://cxcbiology.wordpress.com/tag/cells/
https://cxcbiology.wordpress.com/tag/cells/
You may want to refer to this related Socratic question:
What do the terms hypertonic, hypotonic, and isotonic mean?

