Question #b699a
1 Answer
Allotropy describes the ability of an element to exist in different forms.
Explanation:
Allotropy describes the ability of an element to exist in different forms.
The word originates from Greek, allos, meaning "other", and tropos, "manner".
Allotropy can only be used for forms of an elemnt within the same phase.
A common example of allotropy is with carbon's allotropes.
Carbon exists in many different forms. The most recognized of these forms include diamond, graphite, and soot (amorphous carbon).
At the molecular level, particles of carbon form bonds with other carbon atoms. The arrangement and orientation of these bonds causes the compound to differ in form.
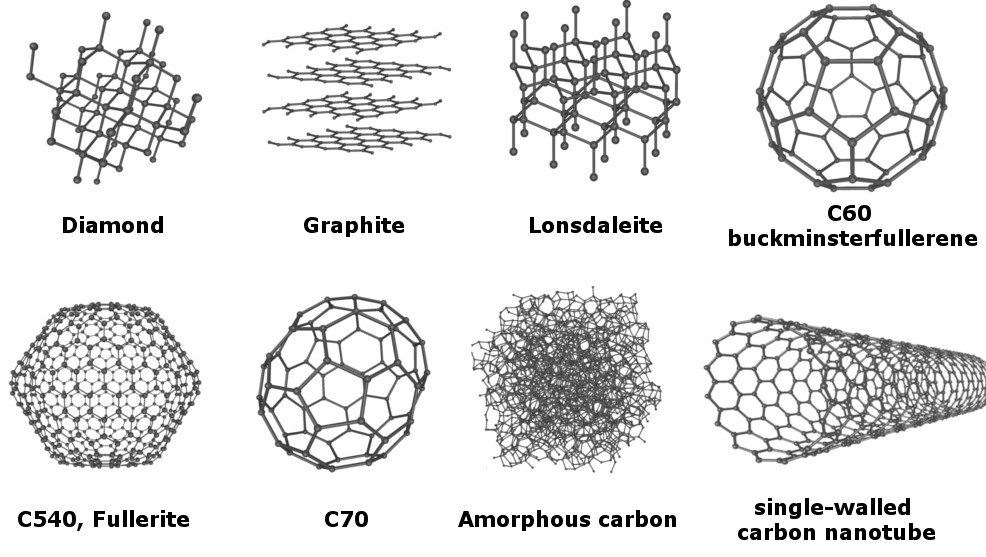
For example, diamond has an orientation of a three-dimensional lattice, which is why diamond is the hardest natural compound.
On the other hand, we have graphite which is arranged in sheet-like hexagons. As you write, each layer is removed.
Hope this helps :)

