Question #ed6cc
3 Answers
Explanation:
The proton-electron balance are what determine the charge of an atom or ion. Protons are positively charged, and electrons are negatively charge, each of ONE unit. Thus, the charge is always the difference between the number of protons and electrons.
More protons than electrons means that it will have a positive charge. More electrons than protons means it will have a negative charge.
Explanation:
Overall charge is the sum of the proton and electrons in an atom.
I will use positive values to represent protons and negative numbers for electrons.
Therefore,
With an overall charge of
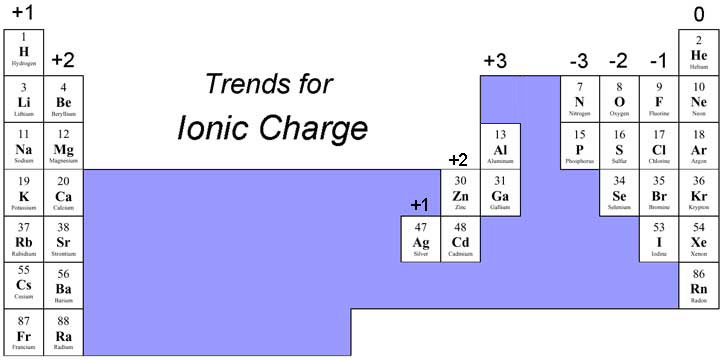
According to the periodic table, only 3 elements have an overall charge of
Hope this helps :)
2-
Explanation:
To find the overall charge of an ion with a number of protons
Charge of ion =
If this is greater than zero, you have a positive ion. If it is less than zero, you have a negative ion.
In the ion mentioned (which from the number of protons appears to be a phosphorous ion), we have
This ion would often be written as



