What is phenolphthalein alkalinity? How is it related to the hydroxide and carbonate levels in water?
1 Answer
See below.
Explanation:
Alkalinity
Alkalinity is a measure of the capacity of water to neutralize acids.
Alkalinity in water is caused primarily to the presence of carbonate, hydrogen carbonate, and hydroxide ions.
Phenolphthalein alkalinity
Phenolphthalein alkalinity is a specific category of alkalinity.
It is determined by titrating to pH 8.3 (where phenolphthalein changes colour).
If you are titrating
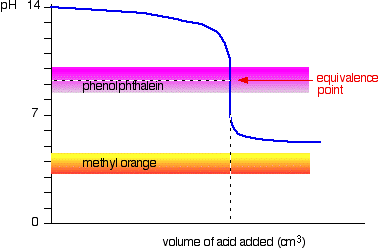
Thus, all of the
If you are titrating
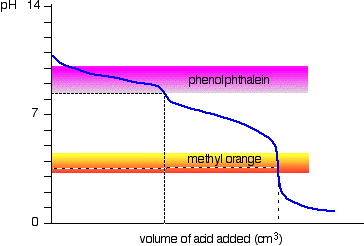
Since
Thus, phenolphthalein alkalinity gives the total hydroxide and half the carbonate level.

